Researchers from the University of Oklahoma have made an exciting advancement that could revolutionize drug discovery and development. The breakthrough centers around a novel technique for adding a single nitrogen atom to bioactive molecules, unlocking a wide array of new possibilities in medicinal chemistry. Published in the renowned journal Science, the research is already attracting significant attention from the global pharmaceutical industry for its potential to streamline drug development and reduce costs.
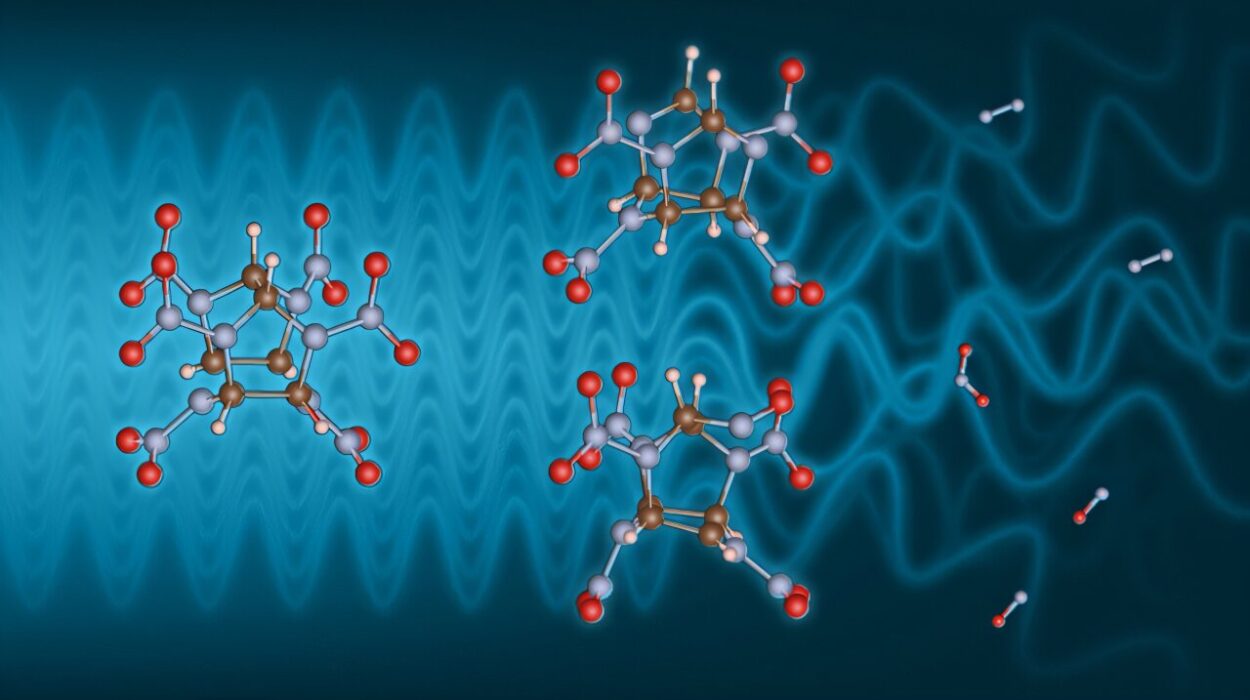
Nitrogen atoms play a crucial role in the structure and function of many important compounds, particularly in pharmaceuticals. The inclusion of nitrogen is particularly significant in the formation of nitrogen-containing chemical structures known as heterocycles, which are common in a large proportion of drug molecules. Heterocycles are fundamental in medicinal chemistry due to their versatility in binding to specific biological targets. The new approach, spearheaded by University of Oklahoma Associate Professor Indrajeet Sharma, utilizes a rare intermediate molecule known as sulfenylnitrene to precisely introduce a single nitrogen atom into existing molecules. This method offers a controlled, efficient way to transform the structure of drug molecules, creating new potential pharmacophores—chemical structures that have therapeutic properties or biological activities that may be exploited for drug development.
The concept, often referred to as “skeletal editing,” is grounded in principles that harken back to the work of Sir Derek Barton, a Nobel laureate who made pioneering contributions to the field of organic chemistry. As Professor Sharma explains, a significant proportion of existing drugs are nitrogen-rich molecules. In fact, about 85% of all FDA-approved drugs contain at least one nitrogen atom, and up to 80% of the top 200 best-selling pharmaceutical products feature nitrogen heterocycles within their chemical structure. The new technique offers a way to further refine these existing molecules by selectively adding nitrogen atoms during the later stages of drug development without altering the molecule’s core functional properties.
By harnessing this capability, drug developers can unlock vast “chemical space,” offering new possibilities for modifying and improving bioactive compounds that would have otherwise been overlooked. Unlike traditional methods, which generally involve developing new compounds from scratch or undergoing extensive chemical transformations, skeletal editing introduces minimal changes, yielding drugs with altered biological and pharmacological characteristics. This is particularly appealing for creating a diverse range of drugs with specific targets or improved efficacy while retaining the characteristics that make the original molecule useful.
The versatility of nitrogen in biological systems, particularly its central role in the structure of DNA, RNA, proteins, and amino acids, makes it a prime target for drug discovery efforts. Introducing a single nitrogen atom can therefore have far-reaching implications, potentially opening the door to new treatments for a range of diseases, including cancer and neurological disorders, which currently remain difficult to treat. In essence, this innovation offers a platform that could influence many critical areas of modern medicine, from cancer therapies to innovations in the treatment of degenerative neurological diseases such as Alzheimer’s and Parkinson’s.
One of the major challenges in drug chemistry has been the use of nitrenes—unstable reactive intermediates that were previously required in these reactions but often resulted in the generation of byproducts that could damage drug molecules. These compounds produced excess oxidizing agents, rendering them incompatible with many biologically relevant compounds. However, the team led by Sharma has overcome this limitation by developing a cleaner method for generating sulfenylnitrenes. These newly developed sulfenylnitrenes are not only metal-free and additive-free but are also compatible with functional groups that are typically seen in drug molecules. This innovation ensures that the skeletal editing process can take place without unwanted side effects, making it an exciting and practical tool for the pharmaceutical industry.
The economic implications of this research are also significant. Drug manufacturing costs can skyrocket depending on the number of synthesis steps required to create a new compound. By incorporating nitrogen into molecules at later stages of development, the complexity of synthesis can be drastically reduced. This “renovation” approach to drug design, as Sharma metaphorically puts it, is akin to modernizing an existing structure rather than building a new one from the ground up. For pharmaceutical companies, this innovation could lower the costs of developing new medications and ensure more affordable prices for consumers, especially when it comes to widely used or essential medications. Given the rising costs of health care, particularly in developed nations like the United States, this new approach holds the potential to decrease the per capita expenditures associated with drug production, ultimately improving access to healthcare.
Sharma further underscores the social implications of this discovery, pointing to how cost reductions could benefit vulnerable populations around the world. While health disparities are an issue globally, certain groups face even greater barriers to accessing medications. Lower drug production costs could result in reduced prices for these populations, making life-saving treatments more accessible and alleviating the financial strain caused by costly pharmaceuticals. This approach is not only a step toward scientific and medical advancement but also represents a potential solution to some of the global health challenges that persist due to limited resources and inequities in healthcare access.
To give context, the cost of health care has been rising globally, with annual health expenditures surpassing $12,000 per capita in the United States alone. The soaring prices of pharmaceuticals play a substantial part in these expenses. Drug manufacturers are increasingly seeking innovative ways to reduce production costs without sacrificing quality. The selective addition of a nitrogen atom in the later stages of drug development offers one such solution. By refining existing molecules rather than starting from scratch, researchers can save time, resources, and reduce overall expenses.
For the pharmaceutical industry, adopting skeletal editing techniques could open the door to the mass production of cost-effective and more diverse drug compounds, accelerating drug discovery processes. Researchers could tailor molecules more efficiently to enhance their therapeutic efficacy while maintaining stability and reducing side effects. With added versatility in medicinal chemistry, many classes of drugs could be optimized for a broader range of applications, meaning more options could be available for the treatment of diseases that currently have limited or no options.
The reaction to this breakthrough has been overwhelmingly positive within both academic and pharmaceutical circles. Its potential to advance pharmaceutical design, alongside a growing focus on making medicines more affordable and accessible to those who need them most, signals an exciting shift toward more sustainable and efficient drug development practices. As the research continues to gain recognition, it will be fascinating to see how this innovative methodology continues to reshape the field of drug development and impact real-world treatment strategies.
Reference: Bidhan Ghosh et al, Sulfenylnitrene-mediated nitrogen-atom insertion for late-stage skeletal editing of N -heterocycles, Science (2025). DOI: 10.1126/science.adp0974