In the mysterious moments between silence and shockwave, something extraordinary happens.
High explosives, like those stewarded at Lawrence Livermore National Laboratory (LLNL), don’t just burst—they transform. Molecules rearrange, bonds rupture, energy surges through their cores. And it all unfolds in mere billionths of a second. Understanding what occurs in those fleeting instants is vital not just for advancing weapons safety, but also for mastering the fundamental science of extreme chemical reactions. Until now, that moment has remained a scientific blind spot—too fast to see, too volatile to measure.
But a new study, published in the Proceedings of the National Academy of Sciences, shines light on this hidden chemistry for the first time. In a landmark collaboration between scientists at SLAC National Accelerator Laboratory and LLNL, researchers have begun to capture the elusive molecular dynamics of explosives during decomposition—opening a potential path to studying the chemistry of detonations in real time.
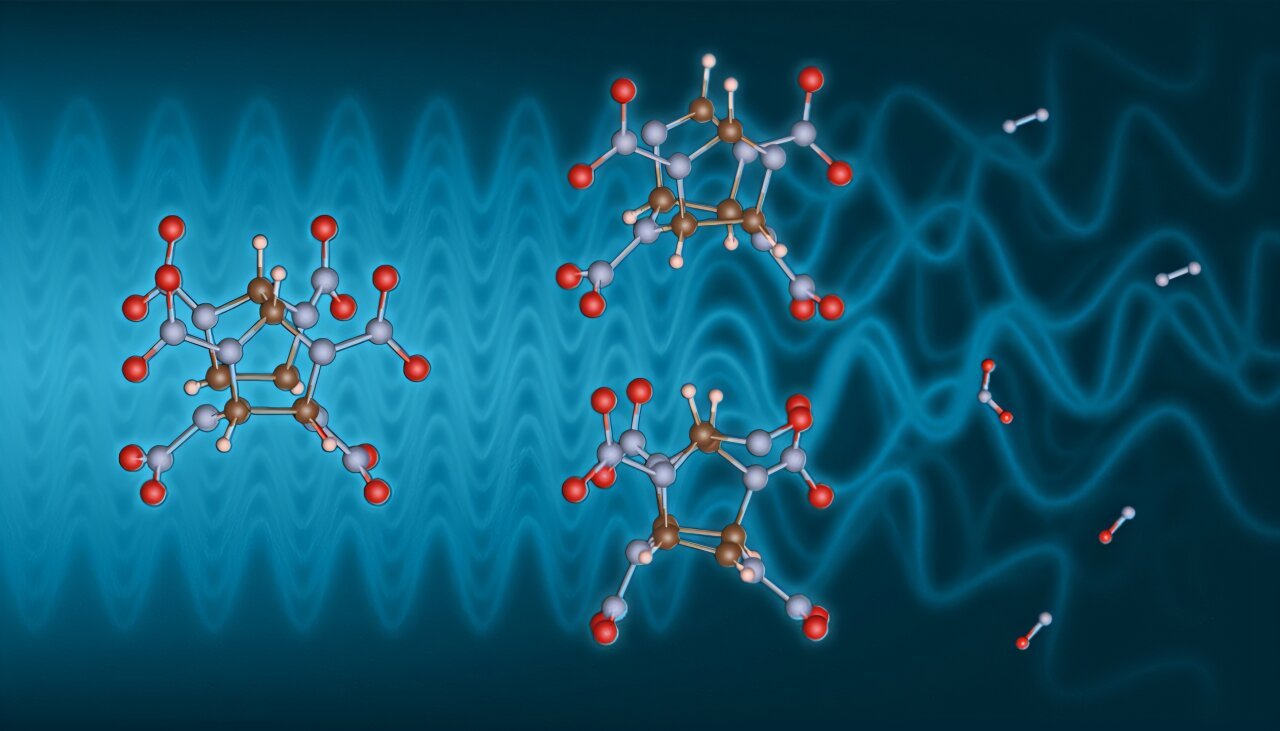
At the heart of this breakthrough is a tool once reserved for probing the cosmic or the crystalline: X-rays. But these aren’t just any X-rays—they’re exquisitely tuned, wielded not just to observe, but to gently coax explosive molecules into action. And under the extreme stillness of cryogenic temperatures, the team caught something never seen before: transient, unstable molecular intermediates—ghosts of the explosion process frozen just long enough to study.
“This work provides the first proof of concept for watching the chemistry of explosives unfold,” said Oscar Paredes Mellone, SLAC scientist and lead author of the study. “We’re finally seeing things that were only theoretical before.”
Lighting the Fuse with X-Ray Vision
Studying explosives is a game of timing. Before detonation, the chemistry is relatively calm—molecules arranged in tight, stable formations. After the blast, only scattered debris remains: molecular soot, chemical char. The in-between—the split-second alchemy of detonation—is a chemical black box.
To unlock it, researchers turned to X-ray Raman scattering, a technique sensitive enough to probe a material’s inner workings without ripping it apart. The method involves firing a stream of X-ray photons at a sample. These photons collide with core electrons in atoms, transferring energy and triggering subtle electronic responses. By measuring the change in energy between incoming and scattered X-rays, scientists can reconstruct a snapshot of the molecule’s structure and composition.
“X-ray Raman scattering is a unique capability that can provide access to the bulk chemistry of these materials,” said Paredes Mellone. Unlike surface-sensitive methods, this technique sees deep into the sample—vital when you’re dealing with dense, tightly packed high explosives.
But the team didn’t just observe. In a clever twist, they used the X-rays themselves to initiate a controlled decomposition of the explosive material. Rather than a violent shockwave, they delivered a precise, slow burn—one that unraveled molecules methodically enough for scientists to measure them as they fell apart.
“It’s not detonation,” emphasized LLNL chemist Trevor Willey, co-author of the study, “but it may be a chemically relevant stand-in. You’re just breaking up the molecules with X-rays instead of a blast.”
Chemistry on Ice: Freezing Explosions in Time
One challenge remained: slowing down a process that naturally wants to go faster than human eyes—or even machines—can track. That’s where cryogenics came in.
By cooling the explosive material to extremely low temperatures, the scientists dramatically decelerated its decomposition. The result? Transient chemical intermediates—fleeting molecular structures that exist for mere instants during a detonation—were effectively frozen in place, allowing researchers to probe them for the first time.
“By doing these much more slow, static, cryogenic experiments, we show that it’s feasible to potentially use X-ray Raman spectroscopy in a detonation experiment,” said Willey. It’s like capturing a high-speed car crash in freeze-frame—not the crash itself, but the early physics of the motion that leads to it.
What they found was revelatory. Using both experimental data and theoretical models, the team identified likely chemical fragments that form during the explosive breakdown. Certain bonds were cleaving apart with precision, causing the internal, cage-like structures of the molecules to open up—a hallmark of detonation chemistry.
To validate their findings, the scientists turned to the National Institute of Standards and Technology (NIST), where they calculated the predicted X-ray Raman signatures of several theoretical intermediates. Comparing these models to real spectral data, they narrowed in on the most probable culprits—chemical ghosts from the first heartbeats of an explosion.
Toward a Real-Time Explosion Microscope
This study is only the beginning. To move from slow, cryogenic experiments to capturing real-time detonations, scientists will need faster tools and more sensitive detectors. But the vision is no longer hypothetical—it’s achievable.
Enter the Linac Coherent Light Source (LCLS) at SLAC—a state-of-the-art X-ray free-electron laser capable of delivering ultra-intense, ultra-fast X-ray pulses. Unlike conventional X-ray sources, LCLS can illuminate reactions in femtoseconds—millionths of a billionth of a second—potentially fast enough to see a detonation as it unfolds, one frame at a time.
“In order to take this dynamic, you would need an incredibly intense and pulsed X-ray source—like the LCLS,” said Willey. “We still need to do a fair amount of work to optimize detectors and improve their efficiency as well.”
That’s already underway. Researchers across SLAC, LLNL, and partner institutions are working to integrate X-ray Raman spectroscopy more routinely at LCLS, developing new techniques and technologies to capture fast chemical dynamics at high resolution.
Why It Matters: Safety, Science, and Stewardship
The implications of this research reach far beyond scientific curiosity.
At LLNL, one of the core missions is stockpile stewardship—ensuring the safety, security, and reliability of the U.S. nuclear deterrent without the need for live testing. To do this, scientists rely on high-fidelity simulations of nuclear and explosive chemistry. But without real experimental data from the detonation zone, those simulations remain partly guesswork.
By capturing the chemical pathways of decomposition and detonation in real-time, researchers could finally validate and refine their models with unprecedented accuracy. That means safer handling, smarter materials, and more reliable designs—not only for defense, but also for applications in space exploration, mining, and materials science.
Moreover, this breakthrough underscores a larger trend: the growing power of photon science to peer into the most extreme, fast, and inaccessible reactions in nature. From planetary cores to battery explosions, the ability to track matter on the atomic scale as it changes—live and in color—is revolutionizing chemistry itself.
The Hidden Symphony of Detonation
In a sense, what the researchers have captured is not destruction, but transformation. The chemistry of explosives, long seen only through its aftermath, is beginning to sing its own song—a symphony of electrons and energy, structure and fracture, order giving way to chaos.
This study is the first movement in a longer composition. But it proves what scientists have long hoped: that even the fastest, most violent reactions in nature can be understood—if you have the right light to see them.
And for the first time, that light has arrived.