Every time you sip a glass of water, you’re drinking one of the strangest, most miraculous substances in the universe. That simple liquid—transparent, tasteless, and often taken for granted—is unlike almost anything else we know. It bends the rules of chemistry, defies expectations, and shapes our planet and our bodies in ways we’re only beginning to fully appreciate.
Why does water behave so strangely? Why does it freeze at 0°C and boil at 100°C—temperatures that seem oddly high for such a small molecule? Why is it densest as a liquid and not a solid? Why can it dissolve more substances than any other solvent?
The answer to all of these lies in one fundamental concept in chemistry: bond polarity.
Understanding bond polarity not only unlocks the secrets of water’s weirdness—it helps us grasp the essential architecture of life itself. In this deep dive, we’ll explore the chemistry of water from the inside out: what bond polarity is, how it gives rise to hydrogen bonding, and why this unique feature has transformed water from a simple molecule into the elixir of life.
The Basics: Molecules, Bonds, and Polarity
To understand water’s special nature, we first need to understand a little bit about how atoms bond and what polarity means.
Atoms form bonds by sharing electrons. When two atoms share electrons equally, the bond between them is said to be non-polar. A good example is the bond between two hydrogen atoms in H₂: they have identical electronegativity (a measure of how strongly an atom pulls on electrons), so they share the electrons evenly.
But not all atoms are equally greedy. When one atom in a bond pulls the electrons closer to itself, the bond becomes polar. This means there’s an unequal distribution of electric charge—a little like a tug-of-war where one team is stronger. One end becomes slightly negative, and the other end becomes slightly positive.
The result is a dipole—a molecule with a positive end and a negative end.
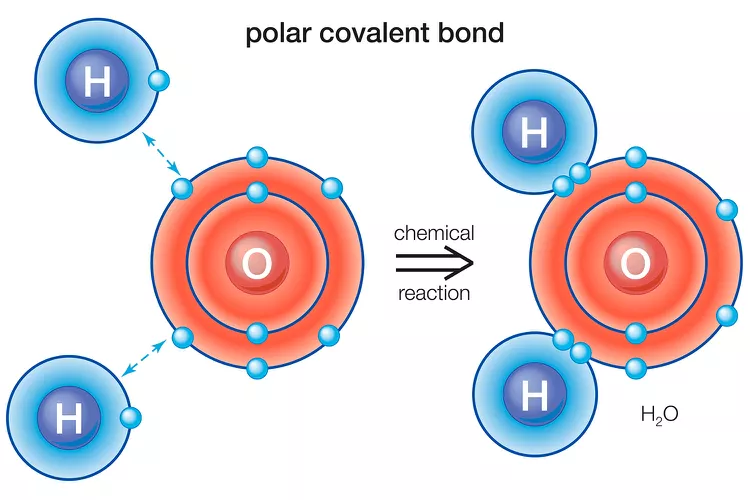
Enter Water: A Molecular Asymmetry That Changes Everything
Water is made up of two hydrogen atoms and one oxygen atom. On paper, H₂O looks simple. But look a little closer and you’ll see why it’s anything but.
Oxygen is much more electronegative than hydrogen. When a hydrogen and an oxygen atom bond, the shared electrons are pulled closer to the oxygen. This creates a polar covalent bond. The oxygen becomes partially negative (δ⁻), and the hydrogen becomes partially positive (δ⁺).
But the real magic happens in water’s shape. Water is not a straight line; it’s bent, with an angle of about 104.5° between the hydrogen atoms. This shape creates an asymmetry: the molecule has a positive side and a negative side—like a tiny molecular magnet.
This molecular polarity—the uneven distribution of charge across the molecule—turns water into a dipolar dynamo, capable of forming an extensive network of bonds with itself and with other molecules.
Hydrogen Bonding: The Invisible Force Behind Water’s Weirdness
Once you have a molecule with a positive and negative end, something astonishing becomes possible: hydrogen bonding.
Hydrogen bonds are not true chemical bonds like covalent or ionic bonds—they’re weaker, more like a magnetic attraction between oppositely charged poles. But don’t let their weakness fool you. In large numbers, hydrogen bonds can have an enormous effect.
In water, each molecule can form up to four hydrogen bonds: two through its hydrogen atoms and two through lone electron pairs on the oxygen atom. These hydrogen bonds constantly break and reform (in liquid water, this happens in trillionths of a second), but together they form a vast, dynamic network that gives water many of its most unusual and life-giving properties.
Let’s explore what those properties are—and why they’re so strange.
Density Anomaly: Why Ice Floats
One of the most surprising consequences of hydrogen bonding is what happens when water freezes.
Most substances become denser as they freeze, because their molecules pack more tightly together. But water does the opposite. When it turns to ice, it becomes less dense—which is why ice floats on water.
Here’s how: as water cools and begins to freeze, its molecules slow down and begin to arrange themselves into a crystalline lattice. This structure is held together by hydrogen bonds, which force the molecules to keep a certain distance from one another.
In this solid form, water’s molecules are actually held farther apart than in the liquid, where they can slip and slide more freely. This “open” lattice is what makes ice less dense than liquid water.
And that’s no trivial fact. Because ice floats, it forms an insulating layer on top of lakes and oceans, protecting the liquid water—and life—below from freezing solid. Without this quirk, entire aquatic ecosystems might die off every winter, and Earth’s climate would be unimaginably different.
High Specific Heat: Water, the Great Thermostat
Another remarkable property of water is its high specific heat—it takes a lot of energy to change its temperature.
Why? Hydrogen bonding again.
Before water can increase in temperature, much of the energy added must go into breaking the hydrogen bonds between molecules. That means water heats up slowly and cools down slowly—a trait that helps moderate Earth’s climate and stabilize the internal temperatures of living organisms.
This is why coastal regions tend to have milder temperatures than inland areas. Oceans absorb and release heat over time, acting as a buffer against temperature extremes.
It’s also why your body can maintain a fairly constant internal temperature: water makes up about 60% of your body weight and acts as a built-in thermal regulator.
Universal Solvent: Polarity in Action
If you’ve ever stirred sugar into your tea or watched salt dissolve in a pot of boiling water, you’ve witnessed another one of water’s incredible powers: it’s known as the universal solvent.
Water can dissolve more substances than any other liquid. The reason? Again, its polarity.
When an ionic compound like salt (NaCl) is added to water, the positive and negative ends of water molecules are attracted to the charged ions. Water molecules surround each ion—oxygen faces the sodium (Na⁺) ions, and hydrogens face the chloride (Cl⁻) ions—and pull them apart into solution.
This ability to dissolve a wide range of substances makes water the ideal medium for biochemical reactions. Our blood, cells, and even our saliva are solutions in which critical molecules are dissolved and transported.
Without water’s dissolving power, life as we know it would be impossible. Metabolism, nutrient absorption, cellular communication—all would grind to a halt.
Surface Tension and Capillary Action: Water’s Clinginess
Water doesn’t just interact with other substances; it clings to itself. This cohesion, a result of hydrogen bonding, leads to surface tension—the force that allows water to form droplets, and insects like the water strider to “walk” on water.
Water also sticks to other materials through adhesion, and together these forces enable capillary action—the movement of water through narrow spaces without the aid of external forces.
Capillary action is how water climbs up a paper towel, or how plants draw water from their roots to their leaves against gravity. It’s vital for everything from ink flowing in pens to blood moving through capillaries.
Once again, bond polarity underpins these forces, guiding the behavior of water molecules with unrelenting precision.
Water and Life: The Role of Polarity in Biology
At every level of biological organization, water’s polarity is critical.
Inside cells, cell membranes are made of phospholipid bilayers—structures with hydrophilic (water-attracting) heads and hydrophobic (water-repelling) tails. This arrangement only works because of water’s polar nature.
Proteins fold into complex shapes based on how different regions interact with water. DNA strands stick together via hydrogen bonds between base pairs—bonds made possible by polar interactions.
Enzymes, receptors, hormones, neurotransmitters—every molecule that sustains life interacts with water in ways dictated by its charge distribution. Without polarity, there is no molecular recognition, no folding, no signaling. Life is not just supported by water—it is structured by it.
Evolutionary Implications: Why Water May Be Universal
One of the most exciting questions in astrobiology is whether water is essential to life across the universe.
Many scientists argue that water’s polarity makes it uniquely suited to support life:
- It can dissolve a wide array of molecules.
- It supports hydrogen bonding for self-assembly of biomolecules.
- It has a wide liquid temperature range.
- It moderates temperature and supports homeostasis.
While some speculate that other solvents like methane or ammonia might support exotic forms of life, most agree that water’s physical and chemical properties—especially its polarity—make it the best candidate.
In other words, Earth’s reliance on water may not be an accident. It might be a cosmic norm.
A Planet Shaped by Water
Water hasn’t just enabled life—it’s shaped the planet itself.
Rain wears down mountains and feeds rivers. Oceans regulate climate. Glaciers carve valleys. Even the very appearance of our blue planet from space—covered in vast oceans—is a testament to water’s prevalence and influence.
Plate tectonics, atmospheric pressure, weather systems—all are intimately tied to water. And all are shaped by the behavior of H₂O molecules responding to one another through polar forces.
From the rain that nourishes crops to the tears that fall in joy or sorrow, water moves through our lives as a silent architect of existence.
Conclusion: The Hidden Power of Polarity
Water is so familiar, so ever-present, that we often forget how strange it really is. Its boiling point is unusually high. Its solid form floats on its liquid. It climbs trees. It shapes weather, erodes rock, and forms the basis of every living cell.
All of this, from the mundane to the miraculous, stems from one elegant feature: bond polarity.
That tiny skew in electric charge across the H₂O molecule unleashes a cascade of effects, from hydrogen bonding to solvation to surface tension, which together turn water from a simple molecule into the linchpin of life.
We live in a universe governed by physical laws and chemical rules. But sometimes, the simplest molecule can reveal a complexity and beauty that borders on the miraculous. Water, in all its flowing, freezing, boiling, dissolving brilliance, is one such miracle.
And it all starts with a little bit of imbalance—a tiny tug-of-war between atoms—that creates the very essence of what we call home.