In a bustling lab at the Max Planck Institute for Molecular Genetics, scientists have developed a molecular tool with a name as bold as its potential: the killswitch. But this is no harbinger of destruction—it’s a precision-engineered synthetic micropeptide designed to freeze specific proteins inside the cell’s most enigmatic compartments, known as biomolecular condensates.
These condensates are like hidden rooms in a house with no walls—compartments without membranes where vital cellular chemistry takes place. Despite their importance in everything from normal cell function to cancer and viral infection, condensates have long resisted detailed study. Now, with the help of this tiny but powerful molecular switch, scientists are turning the lock.
Published this week in Nature, the study marks a milestone in molecular biology and biomedical research. It offers, for the first time, a highly targeted way to manipulate natural condensates inside living cells—without destroying them or flooding the cell with unnatural amounts of proteins.
The Hidden Architecture of the Cell
To the naked eye, a cell looks like a chaotic swirl of jelly. But zoom in with the right tools, and you’ll find an intricate universe of compartments. Among the most mysterious are biomolecular condensates—droplet-like structures where proteins and RNA gather to carry out crucial tasks. These include the nucleolus, which helps make ribosomes; nuclear speckles, which process gene transcripts; and chromocentres, involved in DNA packaging.
Unlike organelles such as the nucleus or mitochondria, condensates don’t have a membrane. They form and dissolve through the principles of phase separation—much like oil droplets forming in water. Their fluid nature allows molecules to enter, exit, and react with astonishing speed and precision.
But probing these mysterious droplets is hard. Traditional methods either dissolve them completely, wiping out their functionality, or require artificial overexpression of proteins that don’t naturally belong. Both approaches distort the very behaviors researchers want to observe.
That’s where the killswitch enters the scene.
A Precision Freeze Frame
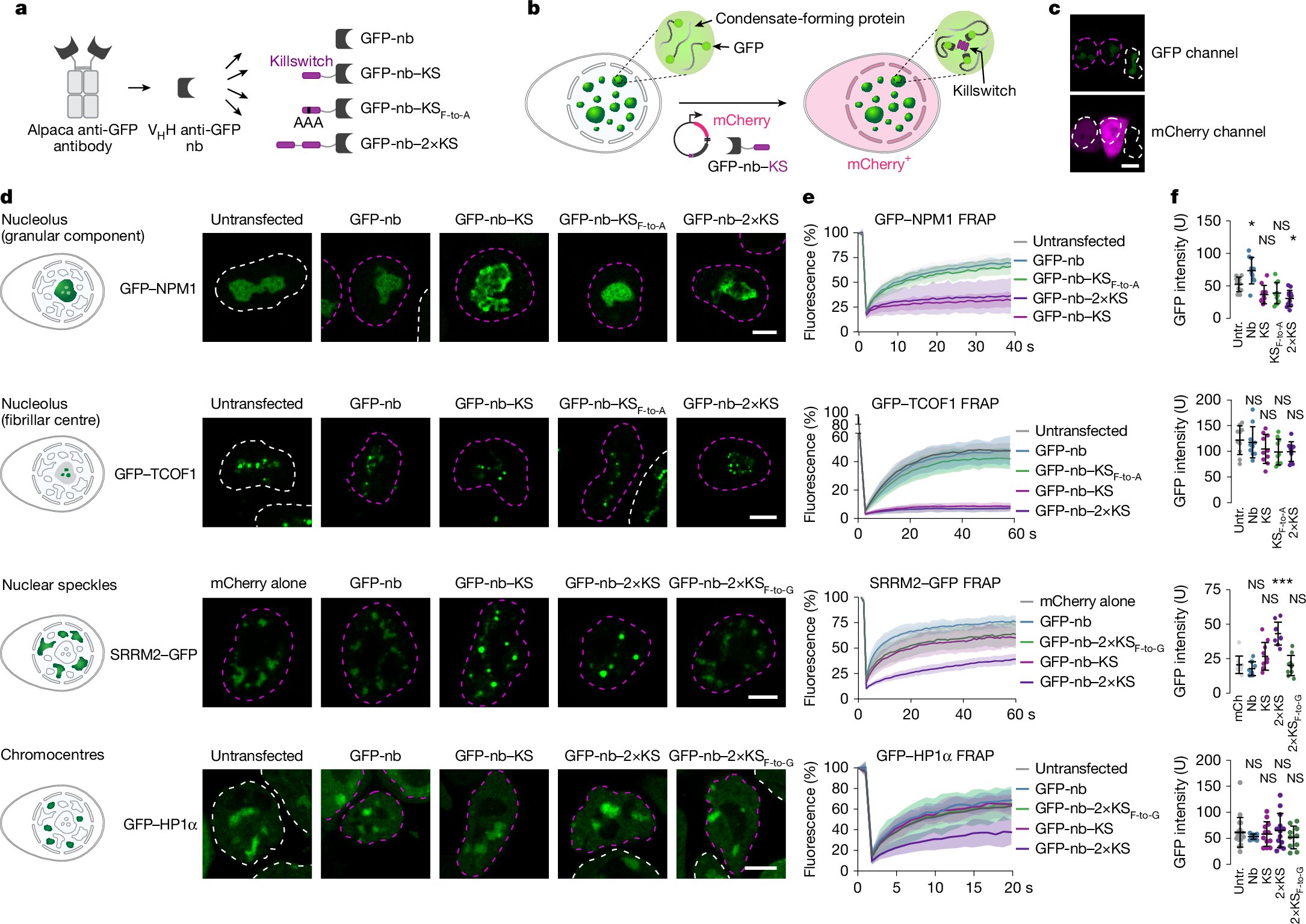
The killswitch micropeptide is a short, hydrophobic strand of amino acids, small enough to slip into condensates but potent enough to bind and immobilize specific proteins. It doesn’t destroy the condensate—it simply freezes part of it in place, like pausing a dance mid-motion to study its choreography.
To direct the killswitch precisely where needed, the team at Max Planck tagged it to small antibody fragments that recognize fluorescent protein markers—like green fluorescent protein (GFP)—already used to label target proteins in living cells. This design gave them pinpoint accuracy in guiding the killswitch into specific condensates.
Starting with human osteosarcoma cells, the team expanded to multiple cell types and condensate varieties, both normal and pathological. They targeted nucleoli, nuclear speckles, chromocentres, and even disease-related condensates formed by fusion oncoproteins in cancer and viral proteins in infected cells.
Watching Proteins Stand Still
To measure the effects, researchers used a technique called fluorescence recovery after photobleaching (FRAP). In this method, part of a fluorescent condensate is bleached with light, and scientists watch how quickly it recovers—an indicator of how mobile its molecules are.
When the killswitch was deployed, the recovery slowed dramatically. In nucleoli, for instance, the movement of key ribosomal proteins was curtailed. Mass spectrometry further revealed that some proteins were excluded altogether, disrupting the normal balance inside the condensate.
The result wasn’t just molecular freeze-frame; it was functional interference. By tweaking the internal environment of these droplets, the researchers could alter their behavior—an insight that could ripple across fields from cancer biology to virology.
Striking Cancer at Its Core
Some of the most eye-opening results came from studying cancer-linked condensates. In acute myeloid leukemia (AML), certain cancer cells are driven by fusion proteins—abnormal hybrids of two genes. These fusion proteins form their own condensates that help maintain the cancerous state.
In a mouse model of AML featuring the fusion protein NUP98::KDM5A, the killswitch didn’t just slow down protein movement—it slowed down the cancer itself. Leukemic cell proliferation dropped sharply, suggesting that manipulating condensate dynamics could be a new therapeutic avenue.
By targeting the unique condensates these fusion proteins form, researchers may one day be able to dismantle cancer’s molecular command centers—without harming normal cells.
Freezing the Machinery of Viruses
The killswitch’s talents don’t end with cancer. The team also explored viral condensates formed by adenovirus protein 52K, which helps assemble viral particles inside infected cells. When the killswitch was directed into these viral condensates, something remarkable happened: they stopped functioning.
With their internal environment scrambled, the viral condensates failed to gather the structural proteins they needed. Viral production dropped by more than 90%.
This suggests the killswitch might someday play a role in antiviral therapies, halting viruses by jamming the very factories they build inside our cells.
A New Era in Cellular Control
What makes the killswitch so promising is its universality. Unlike previous methods that required customized genetic engineering or brute-force chemical interventions, this micropeptide strategy is adaptable, gentle, and precise. It allows researchers to manipulate native proteins in their natural context, without triggering a cellular panic.
“We finally have a tool that lets us ask really nuanced questions about how condensates work,” said one of the study’s senior authors. “It’s like being able to pause a conversation in a crowded room and ask, ‘What did you just say?’—without kicking everyone out of the building.”
The implications are vast. Scientists can now begin mapping how the microenvironments inside condensates shape cellular behavior—and how those environments go awry in disease. They can investigate how these droplets control everything from gene expression to immune response, and eventually, how to engineer condensates with new, therapeutic functions.
The Future of Cell Biology, Frozen and Illuminated
In every living cell, there is order beneath the chaos—a choreography of molecules in endless motion. Until now, that motion has made it hard to study the smallest, most delicate details. But with the advent of the killswitch, researchers have a new kind of microscope—not one that sees, but one that freezes.
By halting proteins mid-step inside their membraneless homes, scientists are opening a new window into how life is built, sustained, and sometimes broken.
And perhaps, someday soon, how it can be healed.
References: Yaotian Zhang et al, Probing condensate microenvironments with a micropeptide killswitch, Nature (2025). DOI: 10.1038/s41586-025-09141-5
A ‘killswitch’ peptide solidifies protein droplets in living cells, Nature (2025). DOI: 10.1038/d41586-025-01875-6