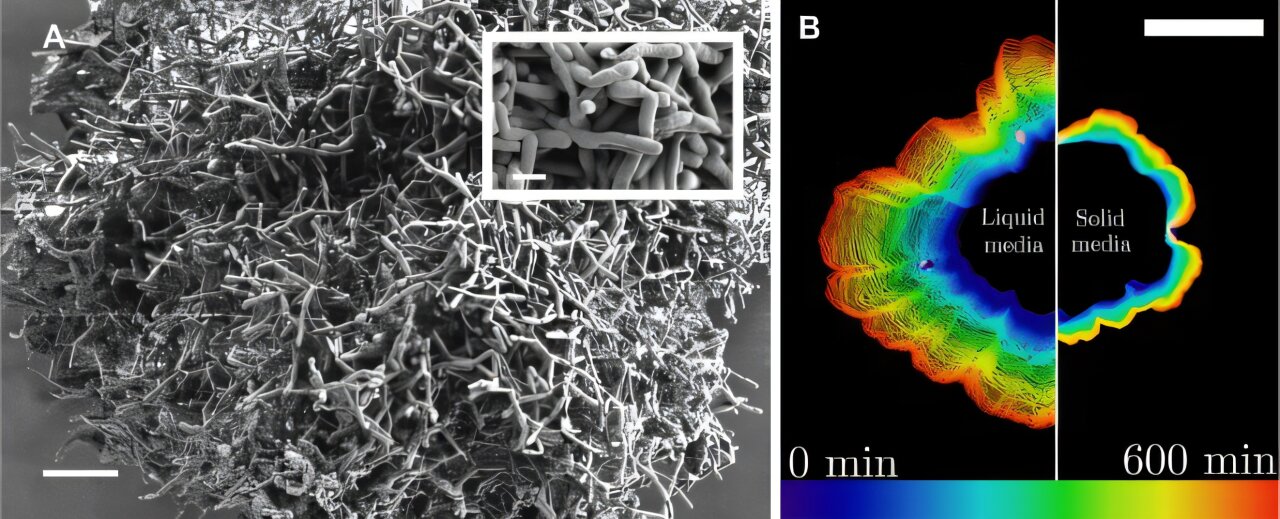
In the quiet hum of a lab, something extraordinary is happening in a petri dish. Tiny yeast clusters—mere blobs of cells floating in warm, sugary liquid—are doing something no one expected. Without flagella, cilia, or complex vascular systems, they are generating their own fluid flows—currents strong enough to ferry nutrients deep into their hearts, breathing life into cells that diffusion alone can’t reach.
This discovery, led by researchers from the Georgia Institute of Technology and India’s National Center for Biological Sciences, sheds new light on one of life’s oldest questions: how did the first multicellular organisms grow large before they evolved the tools to support their bulk?
Published in the journal Science Advances, the study unravels a story of survival and self-organization—a story that began billions of years ago, in a world where complexity was still finding its footing.
The Hidden Challenge of Growing Big
Becoming multicellular is a monumental leap in the history of life. Evolutionarily, it’s a prize worth chasing. Larger size means protection from predators, more access to resources, and the ability to do things single cells simply can’t—like swim with purpose or reach the surface of the sea.
But there’s a catch.
As a cluster of cells grows, its interior becomes harder to feed. Diffusion—the passive movement of nutrients—is efficient only across short distances. In clusters larger than a few hundred micrometers, nutrients can’t reach the cells in the core fast enough. The result: starvation at the center, and stunted growth.
Many modern multicellular organisms have evolved clever ways to solve this: cilia to stir fluid, networks to shuttle food, or even channels that reshape themselves for better access. But early life didn’t have these luxuries. Which begged the question—how did the earliest clusters escape the diffusion trap?
That’s where yeast comes in.
Yeast: A Window Into Evolution’s First Steps
The team studied Saccharomyces cerevisiae, a species of yeast commonly used in baking and brewing, but also in experiments on evolution and development. Using experimental evolution techniques, they encouraged these yeast to grow into spherical clusters in nutrient-rich liquid media, mimicking the conditions of ancient aquatic environments.
Over 12-hour intervals, they watched the yeast grow under high-resolution time-lapse microscopes. The imaging setup was specially designed, with mirrored chambers allowing simultaneous views from multiple angles.
But what they saw wasn’t just slow swelling or outer-layer expansion. Instead, they saw motion—circular, swirling flows inside and around the clusters.
Like miniature whirlpools, the yeast drew fluid in from the sides and pushed it out from the top, creating three-dimensional circulation patterns. When visualized with fluorescent tracer beads, these flows weren’t subtle. They moved with speeds comparable to those produced by highly evolved microorganisms like Volvox and Stentor, which use coordinated beating of cilia to manipulate fluid.
Yet the yeast had no such machinery. So where did these currents come from?
Life Creates Motion from Within
To uncover the source, researchers flipped their experimental chambers upside down—and the flows flipped direction. That pointed to a gravitational driver. But gravity doesn’t act alone. Further experiments ruled out factors like evaporation or surface tension.
What remained was something more profound: buoyant convection, triggered by metabolism itself.
As the yeast metabolized glucose, they consumed sugar and oxygen while releasing ethanol and carbon dioxide—changing the local density of the fluid around them. These subtle shifts in density created gradients that made the fluid move. The yeast had become tiny density pumps, generating circulation simply by being alive.
The flows only appeared when clusters exceeded a critical size—and only if the glucose concentration was high enough. If large clusters were broken into smaller ones, the flow vanished. If smaller clusters were reassembled into a larger whole, it reappeared.
Each cluster behaved like a self-contained organism with its own flow field. When placed close together, neighboring flows would interact, creating interference zones and stagnation points where currents slowed or reversed.
In essence, these clusters had discovered a loophole in physics—a way to sidestep the limitations of diffusion using nothing but their own chemical energy.
A Precursor to Complexity
The implications stretch far beyond yeast. These metabolically driven flows could represent a universal physical mechanism, available to the earliest multicellular organisms on Earth before evolution crafted more sophisticated transport systems.
“It’s a kind of biophysical scaffold,” the authors write—a temporary structure built not from genes but from the inherent laws of fluid dynamics, powered by life’s metabolism. It allows simple organisms to grow exponentially larger than they otherwise could, creating space for more cells, more complexity, and eventually, new functions.
This scaffold may have been the stepping stone that allowed evolution to buy time—letting larger organisms survive long enough to evolve the genetic instructions for blood vessels, lungs, guts, and everything else.
A Glimpse Into Life’s Blueprint
For now, the study stands as a vivid reminder that life doesn’t always need blueprints to solve problems. Sometimes, nature improvises—using the tools already at hand in unexpected ways.
And sometimes, those improvisations change the rules of what’s possible.
The next steps for the research team involve exploring whether similar flows exist in other simple multicellular systems, from bacterial colonies to early-stage embryos. There may be other organisms, other mechanisms, and other invisible dynamics at play—hidden in the quiet eddies of early life.
Back in the lab, the yeast continue to swirl and spin, driven by nothing more than sugar and time. But in their motion, we glimpse something deeper: the earliest whispers of intelligence in nature—not in the mind, but in the physics of living matter.
A reminder that life finds a way—not just through genes, but through flow.
Reference: Nishant Narayanasamy et al, Metabolically driven flows enable exponential growth in macroscopic multicellular yeast, Science Advances (2025). DOI: 10.1126/sciadv.adr6399