Long before lungs drew breath or eyes caught the shimmer of starlight, Earth was a swirling, violent chemistry set. The surface roiled with heat and gas, water crashed against volcanic rock, and the planet’s crust bubbled with raw elements. There were no hearts, no minds, not even cells—just an unceasing dance of atoms in oceans of possibility.
And then, something changed.
Somewhere on this rocky, prebiotic Earth, a line was crossed—a line that would separate the living from the nonliving forever. No one saw it happen. No ancient text recorded the moment. But matter, once lifeless and passive, suddenly started to behave differently. It began to enclose itself, to feed, to respond. It began to evolve.
How life first emerged from chemistry remains one of the deepest, most haunting mysteries science has ever faced. And now, scientists at the University of California San Diego are inching closer to understanding it—not by looking back in time, but by recreating that miracle in a lab.
A New Kind of Cell, Born of Thought
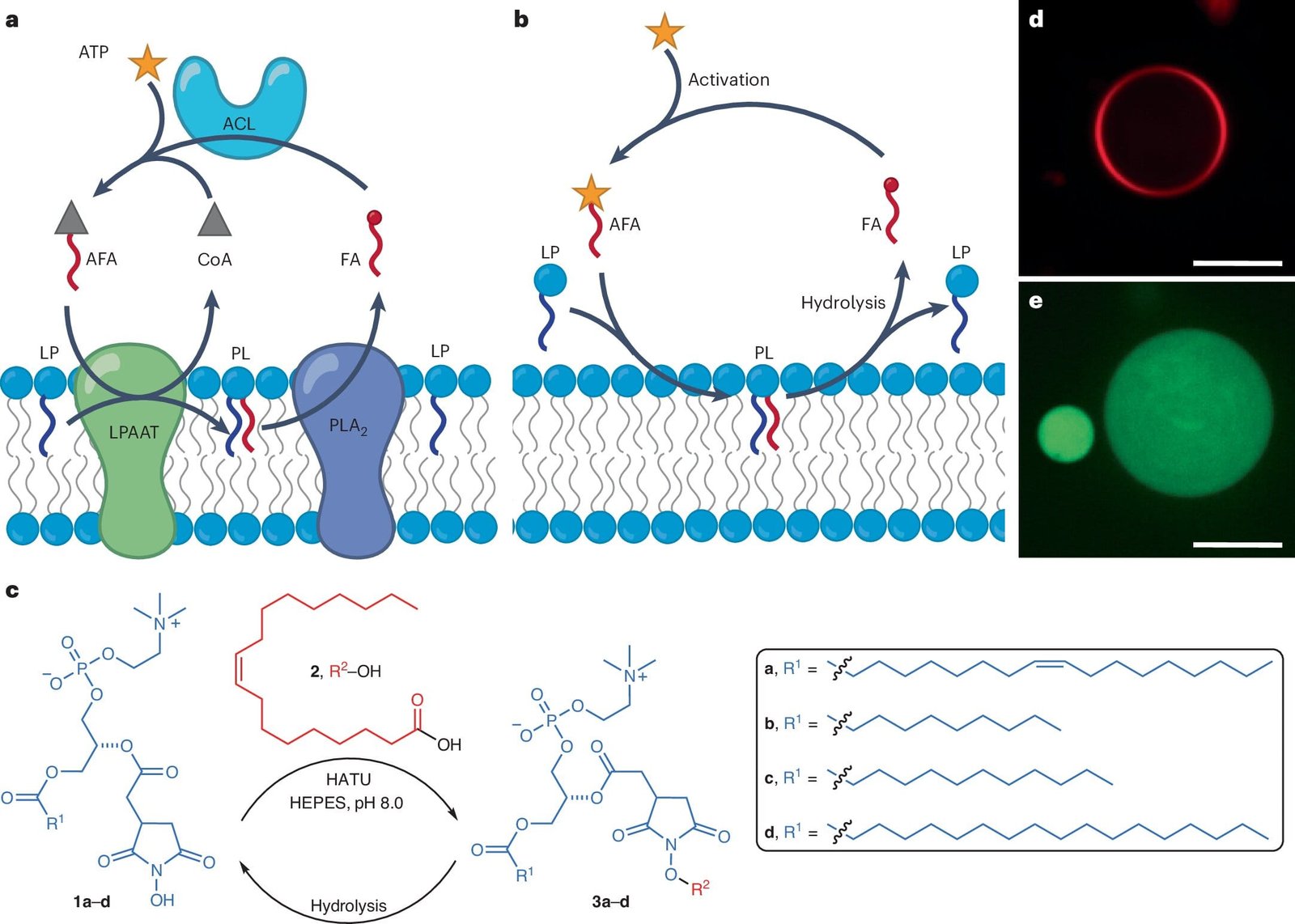
Inside the modern, climate-controlled confines of UC San Diego’s chemistry labs, something remarkable has taken shape. Researchers led by Neal Devaraj and Alessandro Fracassi have created synthetic systems that behave like living cells—cells that can build themselves up and break themselves down, cells that mimic the metabolism at the heart of life.
Their results, published in Nature Chemistry and gracing the cover of the journal’s June 2025 issue, go beyond prior synthetic biology efforts. Where previous attempts focused mostly on building cell-like compartments, this new system adds something far more dynamic: metabolism. It doesn’t just look like a cell—it acts like one, with chemical reactions cycling through growth and decay, order and entropy.
And all of it without a single living molecule.
Life’s Blueprint: Not DNA, But Dynamics
We often imagine DNA as the signature of life. But life, especially in its infancy, was likely something far more primitive. It had no chromosomes, no proteins, no genetics as we know it. What it did have was behavior: the ability to react, to change, to adapt.
Three properties consistently arise in any attempt to define life: compartmentalization, the separation of internal activity from the chaos outside; metabolism, the capacity to transform energy and matter through chemical reactions; and selection, the process that allows some systems to persist while others fall apart.
Until now, most artificial cell research has centered on the first of these—creating bubbles or membranes that enclose molecules and mimic the outer walls of living cells. But without metabolism, these structures are like sculptures: beautiful but static, intricate but inert.
“Cells that lack a metabolic network are stuck,” Devaraj explains. “They aren’t able to remodel, grow, or divide.” In other words, they’re not alive.
What his team has done is give these synthetic cells motion—not mechanical motion, but chemical fluidity. The capacity to assemble, disassemble, and respond. A heartbeat of sorts, made of molecules.
The Dance of Fat and Fire
At the core of this innovation lies a familiar substance: lipids, the fatty molecules that form the membranes of all living cells. In biology, lipids do more than act as barriers; they are architects of motion. They allow cells to merge, split, stretch, and seal. They are the dynamic border patrol of life itself.
Devaraj’s team devised a cycle in which these lipid membranes aren’t just built—they’re also broken down. Their system uses a chemical fuel to activate fatty acids. These energized molecules then link with others called lysophospholipids, creating full phospholipids—the very molecules that naturally organize into membrane structures.
But here’s the twist: when the fuel runs out, the membranes don’t just persist. They degrade. The process reverses, phospholipids unravel, and the system resets. This ebb and flow, this birth and decay, is a simplified form of what metabolism looks like at its most fundamental level.
What makes this so powerful is that the entire process is abiotic—built from nonliving matter. It suggests that life doesn’t need a divine spark or a miracle ingredient. It just needs the right conditions for chemistry to become self-reinforcing.
What Counts as Alive?
This work forces us to ask an uncomfortable question: where exactly does life begin?
If a synthetic cell can fuel its own growth and disassemble when the fuel is gone—if it can mimic the essential rhythms of biology—does that count as life? Or is it still just a machine made of molecules, pretending to be something it isn’t?
Fracassi, the paper’s first author, puts it more pragmatically. “We’re trying to recreate a primitive yet functional cell, one layer at a time.”
The goal is not to build a living organism out of test tubes, but to understand what minimal features make something feel alive. What’s the smallest possible system that can grow, react, and maybe even evolve?
It’s not just a scientific curiosity—it’s a philosophical challenge. Life, it turns out, may not be a thing we are, but a process we follow. And processes can be recreated.
A Glimpse into the Distant Past
These synthetic systems don’t just hint at future applications. They also serve as time machines, letting us peek into Earth’s deep past. Before RNA, before enzymes, before cellular complexity, our planet was a hotbed of chaotic reactions.
Could these lipid cycles have occurred in the wild? Could chemical fuels have been present in volcanic vents or shallow tidal pools? Could life have begun not with a spark, but with a rhythm—a simple, repetitive cycle of assembly and disassembly?
The system developed by Devaraj’s team helps us imagine that world. A place where bubbles formed and popped, where molecules tried combinations at random, where failure was frequent but persistence was rewarded. A world where, one day, a lucky configuration began to continue.
This isn’t the story of a single molecule creating life. It’s the story of billions of tiny chances, running like experiments across oceans and eons.
Why It Matters Today
Though the lab-built cells are primitive compared to the dazzling complexity of modern organisms, their implications are immense. Beyond answering philosophical questions about origins, synthetic cells could transform medicine, industry, and environmental science.
Imagine drug delivery systems that behave like living cells—able to sense their surroundings, regulate their behavior, and respond to conditions inside the human body. Picture artificial biosensors that mimic cellular decision-making, able to detect toxins or pathogens with the subtlety of life itself. Envision systems that clean pollutants or manufacture materials by mimicking the way cells produce proteins or lipids.
All of this is still distant. Devaraj is clear about that. “We may not see these kinds of advancements for 10 or 20 years,” he says. “But we have to do the work today, because we still have so much to learn.”
Rewriting the Genesis
The more we understand about life, the more mysterious it becomes. We now know that life doesn’t require all the bells and whistles of modern biology. DNA, proteins, and organelles may be latecomers to the party. The real engine of life—the part that gives rise to complexity—might be as simple as a loop of reactions fueled by the environment.
The artificial cells built at UC San Diego are not alive in the way you and I are. They don’t think, feel, or grow old. But they breathe, in their own way. They rise and fall, build and decay. And that’s more than we’ve ever achieved with nonliving matter before.
Maybe one day we’ll reach a point where these artificial systems cross another line. Where metabolism gives rise to replication. Where chemistry becomes not just like life—but life itself.
Until then, we’re watching from the edge of the cliff, staring into the deep past and the far future, trying to understand how it all began—and where it might go next.
Reference: Alessandro Fracassi et al, Abiotic lipid metabolism enables membrane plasticity in artificial cells, Nature Chemistry (2025). DOI: 10.1038/s41557-025-01829-5