For over eight decades, a humble white powder called tetra-n-butylammonium bromide hydrate—TBAB·26H₂O—sat in laboratories around the world, playing its quiet role in thermal systems and cooling devices. It was a backstage workhorse, an unsung contributor to air conditioning and energy storage. But within this compound lived a secret so complex that even the sharpest minds in chemistry and materials science couldn’t solve it.
Despite being discovered in 1940, the crystal structure of TBAB·26H₂O remained elusive. Scientists suspected its internal architecture was important—perhaps even revolutionary—for the way it trapped energy. But every time they peered into its molecular skeleton, they were met with ambiguity and contradiction. Some guessed it had a tetragonal lattice. Others suspected a looser arrangement. But guesses are not enough in materials science, where precision dictates performance.
Now, 84 years after it was first synthesized, that mystery has been put to rest. A collaboration between Yokohama National University, the Panasonic Corporation, and Japan’s top synchrotron facility has finally unveiled the true crystal structure of TBAB·26H₂O. The discovery doesn’t just close a long-standing scientific case—it opens the door to a new class of sustainable, water-based materials that could revolutionize how we store and manage heat.
The Ghost in the Crystal
The question that confounded researchers for generations was deceptively simple: how do 26 water molecules wrap around one TBAB molecule to create a semiclathrate hydrate?
Semiclathrate hydrates are part of a broader family of crystalline materials formed when water molecules, bound together by hydrogen bonds, organize themselves into geometric cages around guest molecules. These structures are more than chemical curiosities—they can trap gases, absorb heat, and serve as storage mediums for energy. Nature builds them deep in the ocean to trap methane. Engineers build them to hold coolness until it’s needed.
TBAB·26H₂O was known to behave like a perfect candidate for thermal energy storage. It melted and froze at temperatures suitable for air conditioning, absorbed heat like a sponge, and could be reused over and over again. But unlike its chemical cousins, its exact structure refused to be pinned down. The reasons were complex. The material didn’t lend itself well to X-ray crystallography. Its molecules moved just enough to obscure clean imaging. And the tools available in the 20th century were simply not up to the task.
A Light Brighter Than the Sun
The key to solving the riddle lay not in better guesses, but in better tools. Enter SPring-8—the Super Photon ring-8 synchrotron facility nestled in Sayo Town, Japan. SPring-8 is no ordinary lab. It generates some of the world’s most powerful X-rays by accelerating electrons to near light speed and bending their paths with magnets, causing them to release beams of radiation far more intense than anything found in nature.
These high-intensity X-rays don’t just take photographs of atoms—they reveal their inner conversations. They can track how molecules breathe, shift, and fit together. And in the case of TBAB·26H₂O, they finally gave researchers a way to see what had been invisible for generations.
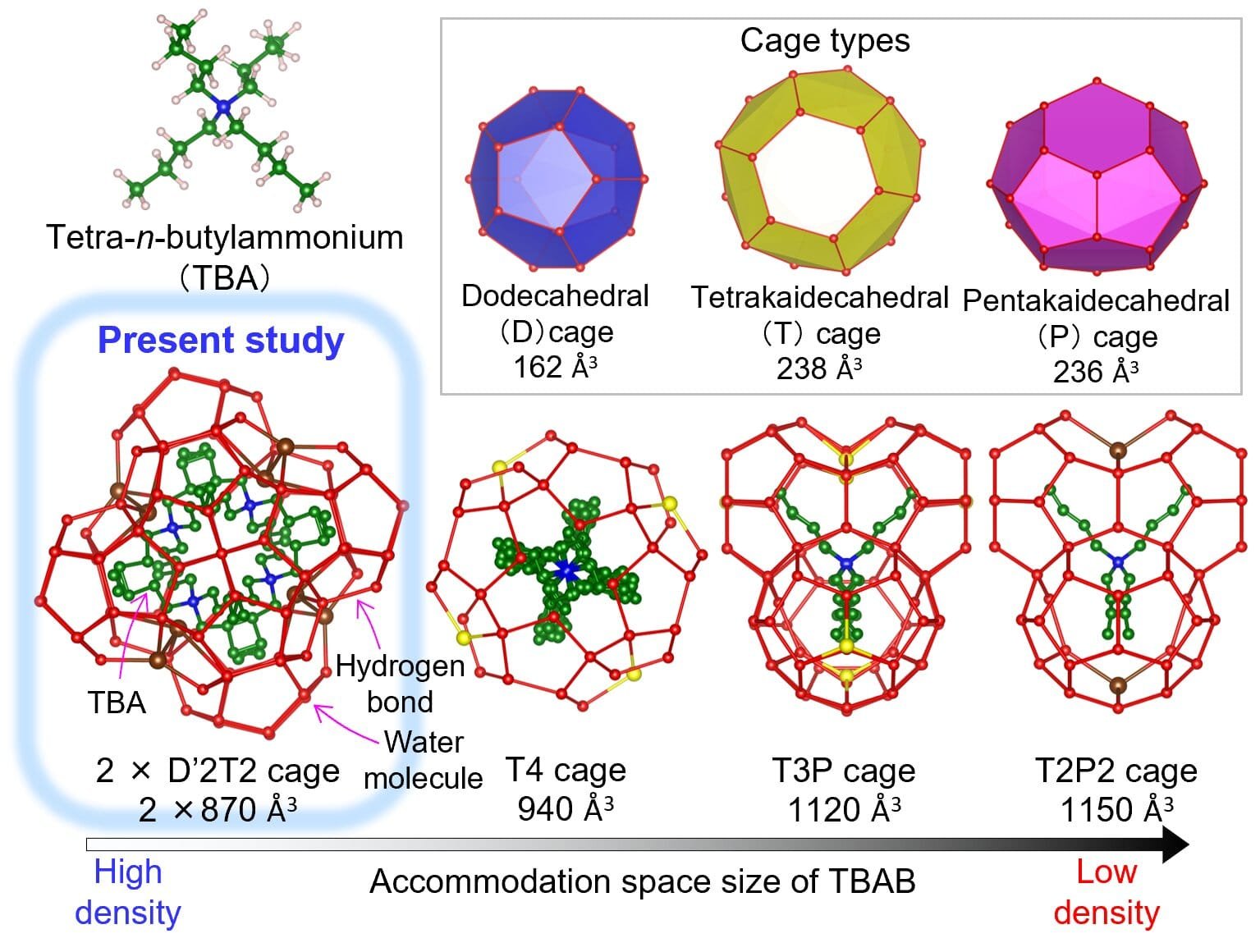
Under the beam of SPring-8, the mystery crystal gave up its secrets. It turns out the structure does have a tetragonal framework, but it isn’t quite what researchers expected. It belongs to a category known as Jeffrey’s Type III hydrates—a rare structural class—marked by intricately arranged cages of water molecules, configured in a way that is both dense and orderly.
The TBAB molecule, a bulky organic ion, fits neatly inside a unique cage formation that hadn’t been observed before in this kind of material. Compared to similar hydrates like TBA(NO₃), the TBAB hydrate’s cage structure is denser and more tightly packed. This denser packing helps explain its superior ability to store and release heat—an insight that had remained out of reach until now.
A Blueprint for Better Cooling
Why does this matter beyond the lab?
Understanding the structure of TBAB·26H₂O turns a mysterious but useful material into a finely tunable technology. With the crystal map now in hand, scientists and engineers can manipulate its properties—its melting point, its stability, its response to environmental changes. They can tweak the size of the cages, substitute guest molecules, or combine it with other materials to improve its function.
Most importantly, this opens new doors in thermal energy storage—a critical field in a warming world.
Today’s air conditioning systems are energy-hungry, and their use is expected to skyrocket as global temperatures rise. TBAB hydrates offer a greener alternative. They can absorb coolness during off-peak hours, store it without electricity, and release it when needed, smoothing out demand on power grids and reducing CO₂ emissions. The better we understand how they work at the molecular level, the more efficiently we can use them.
This discovery also paves the way for applications beyond air conditioning. Hydrates like TBAB·26H₂O could be used in gas separation, carbon capture, and chemical storage—fields where water-based, recyclable materials are desperately needed. Unlike synthetic polymers or rare metals, water is abundant and sustainable. That gives these materials not only practical value but ethical weight in a world looking for low-impact, high-efficiency solutions.
From Puzzling Past to Bright Future
For Sanehiro Muromachi, associate professor at Yokohama National University, and Hironobu Machida, chief engineer at Panasonic Corporation, the success was more than a technical win. It was the culmination of decades of work, the unlocking of a riddle that had defied entire generations of chemists and engineers.
“This structure explains the material’s heat storage characteristics,” Muromachi explained. “It provides new design principles for hydrate-based functional materials.”
Machida added, “Understanding this structure opens the door to engineering better thermal storage and related applications.”
Their voices carry more than pride—they carry vision.
The implications of their discovery are already rippling outward. Other researchers, such as Nobuhiro Yasuda and Hiroyasu Masunaga of the Japan Synchrotron Radiation Research Institute, are building on this breakthrough. And Takeshi Sugahara of the University of Osaka is working to expand these structural insights into soft matter, polymers, and next-generation cooling systems.
The Crystal That Could Help Cool the Planet
In the end, this is a story not just about chemistry, but about persistence and promise.
A crystal that once mocked every attempt to understand it has finally opened its molecular vault. And what we’ve found inside isn’t just atomic geometry—it’s a toolkit for the future. It’s a way to build cleaner, smarter, more sustainable systems. It’s a chance to replace energy-hungry processes with water-based, earth-friendly solutions.
The crystal structure of TBAB·26H₂O no longer sits in mystery. It now stands as a monument to what science can achieve when patience meets precision, when questions outlive careers but not curiosity.
Sometimes the most ordinary-looking materials hold the most extraordinary secrets. And sometimes, solving one small mystery can ripple out to cool an entire world.
Reference: Solving the 80-Year Structure Mystery: Definitive Crystal Structure of TBAB Hydrate Resolved with Synchrotron Radiation, Crystal Growth & Design (2025). DOI: 10.1021/acs.cgd.5c00364