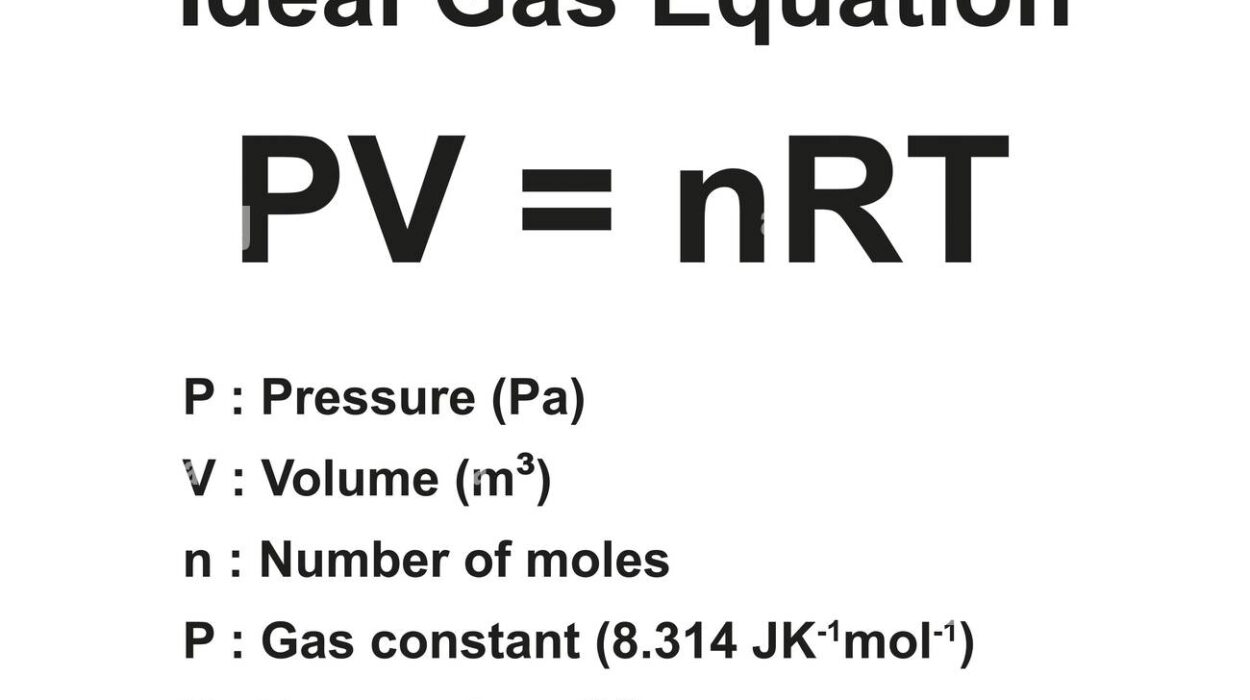
Organic chemistry, the chemistry of carbon compounds, forms the foundation of life as we know it. It is the language in which the structure and behavior of molecules in living systems are written. At the heart of organic chemistry are functional groups—distinct groups of atoms that give molecules their specific chemical characteristics and reactivity. These functional groups are the “personalities” of organic molecules. Whether it’s the pungent smell of vinegar, the sweet scent of bananas, or the toxic punch of cyanide, functional groups are to blame—or to thank.
Understanding functional groups is like learning the alphabet of organic chemistry. They are the key to predicting how molecules will interact, how they will react under certain conditions, and how they can be transformed. In this article, we’ll dive deep into the world of functional groups—not just listing them, but exploring their significance, their behavior, and their role in real-world chemistry, biology, and industry.
So buckle up as we journey through carbon chains and chemical tales, discovering the essential functional groups in organic chemistry that every student, scientist, and curious mind must know.
The Framework of Organic Molecules: Why Functional Groups Matter
Before diving into specific examples, let’s understand why functional groups are so critical. Organic compounds are primarily made up of carbon and hydrogen atoms, often arranged in long chains or rings. On their own, these hydrocarbons are fairly inert—stable, nonpolar, and often unreactive.
Functional groups introduce polarity, reactivity, and chemical character. When a specific arrangement of atoms (like an -OH group) is added to a hydrocarbon backbone, it dramatically changes the molecule’s behavior. That group can attract water, participate in hydrogen bonding, undergo oxidation, or be substituted in a reaction.
In biochemistry, enzymes and hormones derive their specific functions from their functional groups. In pharmaceuticals, tiny changes in a functional group can mean the difference between a life-saving drug and a toxic compound. The shape, charge, and reactivity of functional groups determine how molecules interact with each other and with biological systems.
Now, let’s explore the most essential functional groups and what makes each of them special.
The Hydroxyl Group: The Gateway to Alcohols
The hydroxyl group (-OH) is one of the most common and versatile functional groups in organic chemistry. Molecules that contain one or more hydroxyl groups are known as alcohols. The -OH group is polar due to the electronegativity of oxygen, which pulls electron density away from the hydrogen atom.
This polarity allows alcohols to engage in hydrogen bonding, making them soluble in water and giving them relatively high boiling points compared to similar-sized hydrocarbons. Ethanol (found in alcoholic beverages), methanol (used as a solvent and antifreeze), and glycerol (used in cosmetics and food) all owe their properties to the hydroxyl group.
In reactions, alcohols can be oxidized to form aldehydes, ketones, or carboxylic acids, depending on the type of alcohol and the reaction conditions. They can also participate in esterification reactions to form esters when reacted with carboxylic acids.
In short, the hydroxyl group is a chemical Swiss Army knife—common, reactive, and essential.
The Carbonyl Group: The Power Behind Aldehydes and Ketones
The carbonyl group consists of a carbon atom double-bonded to an oxygen atom (C=O). This group is incredibly important and appears in several different functional groups, including aldehydes, ketones, carboxylic acids, esters, and amides.
In aldehydes, the carbonyl carbon is bonded to at least one hydrogen atom (e.g., formaldehyde, acetaldehyde). In ketones, the carbonyl carbon is bonded to two carbon atoms (e.g., acetone).
The carbonyl group is polar because the oxygen atom is more electronegative than carbon, pulling electrons toward itself. This polarity makes the carbonyl carbon electrophilic, meaning it’s attractive to nucleophiles—species that donate electrons.
Carbonyl-containing compounds are widely used in synthesis. Aldehydes can be oxidized to carboxylic acids or reduced to alcohols. Ketones are slightly less reactive than aldehydes but still participate in a wide range of reactions, such as the nucleophilic addition of alcohols or amines.
In biological systems, carbonyl groups are central to sugar chemistry and are found in the open-chain forms of glucose and fructose.
The Carboxyl Group: Acidity and Reactivity in One Package
The carboxyl group (-COOH) combines a carbonyl group and a hydroxyl group attached to the same carbon. Molecules containing this group are known as carboxylic acids, and they are weak acids capable of donating a proton (H⁺) to solution.
The carboxyl group is found in acetic acid (vinegar), citric acid (citrus fruits), and fatty acids. Its acidity arises from the resonance stabilization of the carboxylate ion that forms when the proton is lost.
Carboxylic acids are highly reactive in organic synthesis. They readily form esters when reacted with alcohols (a process called Fischer esterification) and amides when reacted with amines. They can also be reduced to aldehydes or alcohols under specific conditions.
In biological systems, carboxylic acids are fundamental. Amino acids, the building blocks of proteins, each contain a carboxyl group. The reactivity of this group is central to peptide bond formation and metabolism.
The Amino Group: The Backbone of Proteins
The amino group (-NH₂) consists of a nitrogen atom bonded to one or more hydrogen atoms. When attached to a carbon skeleton, this forms an amine.
Amines can be classified as primary (one carbon attached to nitrogen), secondary (two carbons), or tertiary (three carbons). Their basicity makes them critical in biological systems where acid-base reactions are common.
The amino group plays a key role in nucleophilic substitution reactions and can form hydrogen bonds due to its lone pair of electrons on nitrogen. In pharmaceuticals, many active ingredients feature amino groups that influence how the compound interacts with biological targets.
Amino acids—the monomers of proteins—contain both an amino group and a carboxyl group. The interaction between these two groups is what allows amino acids to link together into long, functional protein chains.
The Ester Group: Fragrance and Flavor
Esters are formed from the reaction between a carboxylic acid and an alcohol, resulting in a compound with the general formula R-COOR′. The functional group of an ester is -COO-.
Esters are responsible for many pleasant scents and tastes. The sweet smell of bananas, the fruity aroma of strawberries, and the scent of pineapples are all due to esters.
Chemically, esters are less reactive than carboxylic acids but can be hydrolyzed back into their original components under acidic or basic conditions. This property is exploited in many biochemical pathways and industrial processes.
In polymers, esters form the backbone of polyesters, a class of materials that includes common fabrics and plastics like PET (polyethylene terephthalate). Their durability, flexibility, and reactivity make esters highly versatile.
The Ether Group: The Gentle Solvent
Ethers contain an oxygen atom connected to two alkyl or aryl groups (R-O-R′). The ether group is relatively nonpolar, does not participate in hydrogen bonding (unless aromatic), and has a low boiling point.
Ethers like diethyl ether were once widely used as anesthetics due to their volatility. Today, they are more commonly employed as solvents in chemical reactions.
Although generally unreactive, ethers can undergo cleavage reactions with strong acids, and under certain conditions, they can form explosive peroxides. Their stability and non-polarity make them ideal media for reactions involving organometallic reagents, like Grignard compounds.
The Amide Group: Stability and Strength
Amides are formed when a carboxylic acid reacts with an amine. The resulting functional group is -CONH₂, or variations thereof depending on the structure.
Amides are incredibly important in biochemistry. The peptide bond that links amino acids in proteins is an amide bond. This bond is stable due to resonance, which gives the nitrogen-carbon bond partial double-bond character, reducing its reactivity.
In organic chemistry, amides are often used in the formation of pharmaceuticals, dyes, and synthetic materials. The famed compound acetamide is the simplest amide, and more complex amides appear in antibiotics like penicillin.
Their stability and specific geometry make amides ideal in molecules where rigidity and planarity are desired.
The Nitrile Group: Sharp, Toxic, and Reactive
Nitriles contain a carbon triple-bonded to a nitrogen atom (C≡N), and they are often associated with sharp, bitter odors.
Although some nitriles are highly toxic—like hydrogen cyanide—others are essential intermediates in organic synthesis. Nitriles can be hydrolyzed to carboxylic acids, reduced to amines, or used in polymerization.
They play a role in the manufacture of synthetic rubber, pharmaceuticals, and agrochemicals. The reactivity of the nitrile group lies in the polarized triple bond, which makes the carbon highly electrophilic.
The Thiol Group: The Sulfurous Cousin of Alcohols
Thiol groups (-SH), also known as mercaptans, are sulfur analogs of alcohols. They are noted for their strong, often unpleasant odors—think skunk spray or rotten eggs.
Despite their smell, thiols play crucial biological roles. Cysteine, an amino acid containing a thiol group, can form disulfide bonds that stabilize protein structures.
Thiols are more acidic than alcohols and can be readily oxidized to form disulfides. In organic synthesis, they are used as nucleophiles and ligands in coordination chemistry.
The Halides: Electronegative and Useful
Halogen-containing functional groups involve a halogen (F, Cl, Br, I) bonded to a carbon atom. These groups are collectively called alkyl halides or haloalkanes.
The presence of a halogen imparts reactivity due to the polarized carbon-halogen bond. Halides are often used as intermediates in substitution and elimination reactions. They are also precursors to Grignard reagents, powerful tools in carbon-carbon bond formation.
Chloroform, carbon tetrachloride, and freon are all examples of halogenated compounds with historical industrial uses, although environmental concerns have reduced their application.
Conclusion: The Language of Organic Chemistry
Functional groups are the grammar of organic chemistry. They define how molecules behave, how they react, and how they interact with other molecules. Knowing these groups is not merely a requirement for passing exams—it is the foundation of understanding the molecular world.
From the simplest alcohol to the most complex pharmaceutical, functional groups allow chemists to predict, design, and manipulate molecules with precision. They make it possible to synthesize life-saving drugs, develop new materials, and unravel the mysteries of biochemistry.
Whether you’re a student, researcher, or simply someone curious about the invisible world around you, mastering functional groups opens a door into one of the most profound languages of nature.
Organic chemistry may be complex, but at its core, it’s built from recognizable, reliable patterns. Learn the functional groups, and you gain the power to see those patterns everywhere—from the food you eat to the cells in your body and the medicines in your cabinet.