In the world of chemistry, isomerism is like a magic trick where molecules—despite having the same chemical composition—can take on dramatically different forms. Just as two people can have the same name but vastly different personalities, isomers are molecules that share the same molecular formula but differ in their arrangement of atoms.
At first glance, this might sound like a trivial distinction, but the implications of isomerism are profound. The physical properties, chemical reactivity, and biological activity of molecules can change entirely based on their structure. Isomerism is, therefore, one of the most exciting phenomena in chemistry, adding layers of complexity to the understanding of molecular behavior and reactions.
In this article, we’ll delve into the concept of isomerism, breaking it down into easily digestible parts, with real-world examples and visual illustrations. Whether you’re a chemistry enthusiast or just beginning your journey into the world of molecules, this article will serve as your guide to the fascinating world of isomerism.
What is Isomerism?
Isomerism refers to the phenomenon where two or more compounds share the same molecular formula but differ in the way their atoms are arranged in space. This results in molecules that may have different structures, chemical properties, or physical characteristics, even though they contain the same number and types of atoms.
The word “isomer” comes from the Greek words “isos,” meaning equal, and “meros,” meaning part. So, isomers are compounds with “equal parts,” but arranged in different ways. There are several types of isomerism, each offering a different kind of variation in molecular structure.
Isomerism is a crucial concept because it explains how different compounds with identical molecular formulas can behave completely differently. From the fuel we put in our cars to the medications we take, isomerism plays a vital role in determining how substances interact with each other and with living organisms.
Types of Isomerism
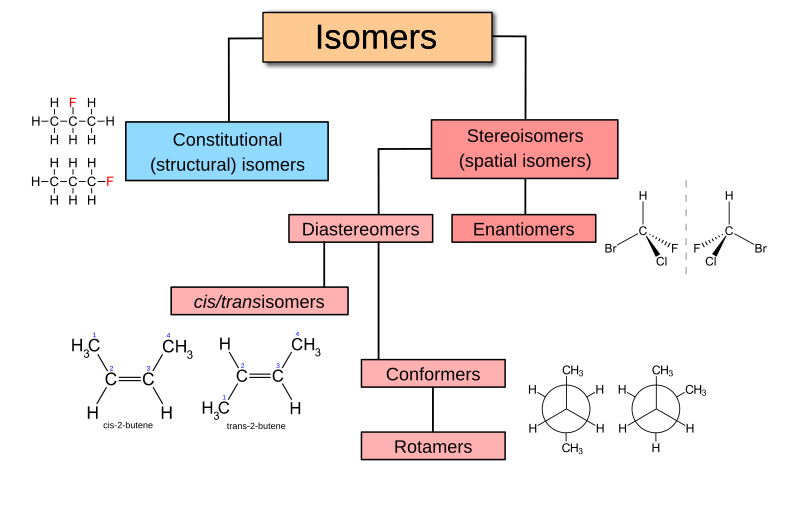
Isomerism can be broadly classified into two major categories: structural isomerism and stereoisomerism. Both types are fascinating in their own right and lead to different types of isomers that exhibit varying behaviors and properties.
1. Structural Isomerism: Different Connections, Same Composition
Structural isomerism, also known as constitutional isomerism, occurs when molecules with the same molecular formula have different bonding patterns. In other words, the atoms in these isomers are connected differently, which gives rise to distinct structures with unique properties.
There are several subtypes of structural isomerism, including:
A. Chain Isomerism
Chain isomerism occurs when the carbon backbone of the molecule is arranged differently. This can involve different branching patterns or the length of the carbon chain itself.
Example: Butane (C₄H₁₀)
Butane, a simple hydrocarbon, can exist as two different isomers:
- n-Butane: This is the straight-chain version, where four carbon atoms are connected in a row (C-C-C-C).
- Isobutane: This is the branched version, where three carbon atoms form a chain and one additional carbon atom branches off (C-C-C).
Both n-butane and isobutane share the same molecular formula (C₄H₁₀), but because their atoms are arranged differently, they have different chemical properties. For example, isobutane has a lower boiling point compared to n-butane, which affects their uses in fuel and refrigeration.
Visual Representation:
- n-Butane: CH₃-CH₂-CH₂-CH₃
- Isobutane: CH₃-CH(CH₃)-CH₃
B. Position Isomerism
Position isomerism occurs when the functional group (like an alcohol group or a halogen atom) is attached to different positions on the carbon chain.
Example: 1-Chlorobutane and 2-Chlorobutane
Both molecules share the same molecular formula (C₄H₉Cl), but the chlorine atom is attached to different positions on the carbon chain:
- 1-Chlorobutane has the chlorine atom attached to the first carbon.
- 2-Chlorobutane has the chlorine atom attached to the second carbon.
The difference in positions leads to slight variations in their physical properties, like boiling points and solubility in water.
Visual Representation:
- 1-Chlorobutane: CH₃-CH₂-CH₂-CH₂Cl
- 2-Chlorobutane: CH₃-CH₂-CHCl-CH₃
C. Functional Group Isomerism
Functional group isomerism arises when different functional groups (like alcohols, ethers, aldehydes, etc.) are attached to the same carbon skeleton.
Example: Ethanol and Dimethyl Ether
Both ethanol (C₂H₆O) and dimethyl ether (C₂H₆O) have the same molecular formula, but they feature different functional groups:
- Ethanol has a hydroxyl group (-OH), making it an alcohol.
- Dimethyl Ether has an ether group (-O-), linking two methyl groups (CH₃).
These two compounds behave very differently: ethanol is a common alcohol used in beverages and solvents, while dimethyl ether is used as a propellant in aerosol cans.
Visual Representation:
- Ethanol: CH₃CH₂OH
- Dimethyl Ether: CH₃OCH₃
D. Tautomeric Isomerism
Tautomeric isomerism is a special kind of structural isomerism where two isomers exist in equilibrium, and the isomerism arises from the migration of a hydrogen atom and a double bond between two atoms. This is common in organic compounds containing functional groups like aldehydes, ketones, and alcohols.
Example: Keto-Enol Tautomerism
A common example of tautomerism is the interconversion between the keto form and the enol form of certain carbonyl compounds:
- In the keto form, the compound contains a carbonyl group (C=O).
- In the enol form, the compound has an alcohol group (-OH) attached to a carbon atom with a double bond.
This equilibrium between the two forms affects the chemical reactivity and stability of the compound. The keto form is typically more stable, but the enol form can play a crucial role in certain chemical reactions, such as in biological systems.
Visual Representation:
- Keto Form: CH₃COCH₂CH₃ (Acetone)
- Enol Form: CH₃C(OH)=CH₂
2. Stereoisomerism: Same Composition, Different Orientation
Stereoisomerism occurs when molecules with the same molecular formula and the same bonding patterns differ only in their three-dimensional spatial arrangement. These isomers are particularly intriguing because they can exhibit drastically different physical and chemical properties due to their different shapes and orientations in space.
There are two main subtypes of stereoisomerism: geometrical (cis-trans) isomerism and optical isomerism.
A. Geometrical Isomerism (Cis-Trans Isomerism)
Geometrical isomerism arises when molecules with restricted rotation around a bond (usually a double bond or a ring structure) can exist in two different forms: cis (same side) and trans (opposite side). These two forms can differ significantly in their physical and chemical properties.
Example: 2-Butene
Consider the compound 2-butene, which has the molecular formula C₄H₈. This compound can exist in two forms:
- Cis-2-Butene: The two methyl groups (CH₃) are on the same side of the double bond.
- Trans-2-Butene: The two methyl groups are on opposite sides of the double bond.
The cis form has a different dipole moment and melting point than the trans form, which affects its behavior in reactions and in physical properties such as boiling point and solubility.
Visual Representation:
- Cis-2-Butene: CH₃-CH=CH-CH₃ (methyl groups on the same side)
- Trans-2-Butene: CH₃-CH=CH-CH₃ (methyl groups on opposite sides)
B. Optical Isomerism
Optical isomerism occurs when a molecule can exist in two non-superimposable mirror-image forms, known as enantiomers. These enantiomers are like a pair of hands—identical in structure but different in orientation.
Enantiomers have the same physical properties (such as boiling point and melting point) but can have different effects in biological systems. For example, one enantiomer of a drug might be therapeutic, while the other could be harmful or have no effect at all.
Example: Lactic Acid
Lactic acid (C₃H₆O₃) is a simple organic acid that has two enantiomers:
- L-lactic acid (L for left-handed) is the form found in our muscles and is produced during anaerobic respiration.
- D-lactic acid (D for right-handed) is used in some bacteria and can be found in fermented foods.
These two forms are mirror images of each other and cannot be superimposed, like left and right hands. The two enantiomers of lactic acid have different effects in the body, with L-lactic acid being more relevant to biological processes in humans.
Visual Representation:
- L-Lactic Acid: HOCH₂-CH(OH)-COOH (left-handed)
- D-Lactic Acid: HOCH₂-CH(OH)-COOH (right-handed)
Why Does Isomerism Matter?
Isomerism is not just an academic curiosity; it has real-world applications that impact everyday life in ways you might not even realize. The distinct properties of isomers can influence everything from the way chemicals are synthesized to how they behave in biological systems.
For example, the difference between enantiomers can make or break a
pharmaceutical drug. The famous example of thalidomide—a drug that caused birth defects when one enantiomer was administered, while the other had therapeutic effects—highlights the importance of stereochemistry in medicine.
In the world of materials science, isomeric forms of compounds can lead to the development of new polymers with unique properties, such as increased strength, flexibility, or resistance to degradation.
From the food we eat to the medicine we take, isomerism plays a crucial role in how substances interact with each other and with the environment. It also provides chemists with the flexibility to design new molecules with specific functions, making it an indispensable tool in synthetic chemistry.
Conclusion
Isomerism is one of the most fascinating and essential concepts in chemistry. It teaches us that molecules, while made up of the same atoms, can exist in many different forms—each with unique properties and behaviors. Whether it’s the difference between straight-chain and branched hydrocarbons, the subtle shifts in spatial arrangement that lead to different reactivities, or the molecular quirks that give rise to life-saving medications, isomerism reveals the complex beauty of the molecular world.
Understanding isomerism opens up endless possibilities in science, technology, and medicine. It’s a concept that continues to drive discovery, innovation, and progress, shaping the future of chemistry and beyond.