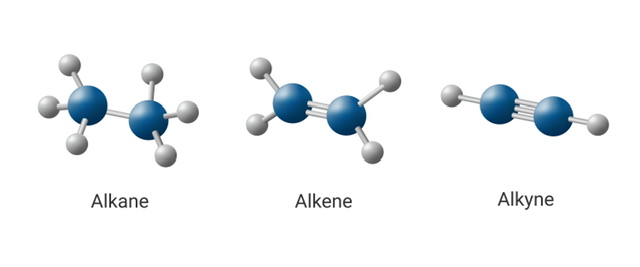
In the world of organic chemistry, the study of hydrocarbons is fundamental. Among the vast array of hydrocarbons, three primary categories stand out for their simplicity and importance: alkanes, alkenes, and alkynes. These three groups of compounds are distinguished by the types of bonds that hold their carbon atoms together—single, double, or triple bonds. Understanding these hydrocarbons not only illuminates the basics of organic chemistry but also provides the foundation for much of modern industry, from fuel production to the creation of plastics and medicines.
While they may sound technical and somewhat intimidating, alkanes, alkenes, and alkynes are fascinating and essential to our everyday lives. Whether it’s the gasoline that fuels our cars or the plastic products we use daily, these compounds form the backbone of numerous materials that shape our world. In this article, we will explore the nature of these hydrocarbons, delve into their structure, properties, and uses, and discover how they play a crucial role in chemistry and industry.
What are Alkanes?
Structure and Bonding of Alkanes
Alkanes, also known as paraffins, are the simplest type of hydrocarbons. They are composed only of carbon (C) and hydrogen (H) atoms, connected by single covalent bonds. Each carbon atom in an alkane is sp³ hybridized, meaning it forms four single bonds in a tetrahedral arrangement. The general formula for alkanes is CnH2n+2C_nH_{2n+2}, where n is the number of carbon atoms.
The most straightforward alkane is methane (CH₄), the primary component of natural gas, which contains only one carbon atom bonded to four hydrogen atoms. As we move up the series, alkanes increase in complexity. For instance, ethane (C₂H₆) has two carbon atoms, each bonded to three hydrogens, while propane (C₃H₈) has three carbon atoms.
Alkanes can be categorized into two types:
- Straight-chain alkanes: These are alkanes in which the carbon atoms are connected in a continuous chain, such as in butane (C₄H₁₀).
- Branched-chain alkanes: These contain branches or side chains of carbon atoms, like isobutane (C₄H₁₀), where one of the carbons in the chain is connected to a branch of other carbon atoms.
Physical Properties of Alkanes
The physical properties of alkanes depend largely on their molecular size and structure. As the number of carbon atoms increases, the molecules become larger, leading to a rise in boiling and melting points. Small alkanes like methane and ethane are gases at room temperature, while larger ones such as octane (C₈H₁₈) are liquids, and even larger ones, like those found in waxes and paraffin, can be solids.
Alkanes are non-polar molecules due to the symmetry of their bonds. As a result, they are generally insoluble in water but are soluble in non-polar solvents like benzene or ether. They are also relatively unreactive, as single bonds between carbon atoms are quite stable. This is why alkanes are often used as fuels—like gasoline, diesel, and natural gas—because they burn cleanly and produce a high amount of energy.
Uses of Alkanes
Alkanes are used extensively in the energy sector. Methane, the simplest alkane, is a primary component of natural gas and is used for heating, cooking, and electricity generation. Propane, another important alkane, is commonly used for heating and as a fuel for vehicles in areas where natural gas pipelines are unavailable. Butane, found in liquefied petroleum gas (LPG), is also a commonly used fuel for heating and cooking.
Alkanes are also integral in the production of synthetic materials, such as plastics and rubber. When they undergo cracking and polymerization processes, they can be transformed into a variety of materials used in everything from packaging to medical devices.
What are Alkenes?
Structure and Bonding of Alkenes
Alkenes are hydrocarbons that contain at least one carbon-carbon double bond. The general formula for alkenes is CnH2nC_nH_{2n}, where n represents the number of carbon atoms in the molecule. The double bond between the carbon atoms is composed of one sigma bond and one pi bond. The pi bond, which forms as a result of the sideways overlap of p orbitals, is weaker than the sigma bond, making alkenes more reactive than alkanes.
The most basic alkene is ethene (C₂H₄), which has two carbon atoms connected by a double bond and each carbon bonded to two hydrogens. Alkenes can also be branched or have cyclic structures, such as in the case of cyclohexene (C₆H₁₀), which has a six-membered ring with one double bond.
Geometrical Isomerism in Alkenes
One of the most interesting features of alkenes is their ability to form geometrical isomers due to the restricted rotation around the double bond. This occurs when two different substituents are attached to each carbon of the double bond. These isomers are classified as:
- Cis-isomers: The substituents are on the same side of the double bond.
- Trans-isomers: The substituents are on opposite sides of the double bond.
For example, in but-2-ene (C₄H₈), the two methyl groups can be on the same side (cis-but-2-ene) or opposite sides (trans-but-2-ene). These isomers often have different physical and chemical properties, such as boiling points and solubility.
Physical Properties of Alkenes
Alkenes have physical properties similar to alkanes, but they tend to be more reactive due to the presence of the double bond. Like alkanes, alkenes are non-polar molecules and are insoluble in water. However, they are more likely to undergo chemical reactions such as addition reactions, in which atoms or groups of atoms add across the double bond.
As the number of carbon atoms increases, the boiling and melting points of alkenes rise. Smaller alkenes, such as ethene and propene, are gases at room temperature, while larger ones are liquids or solids.
Chemical Reactions of Alkenes
Alkenes are much more reactive than alkanes due to the nature of the double bond. The pi bond in the double bond is weaker than the sigma bond, making it susceptible to attack by a variety of reagents. Some of the key reactions of alkenes include:
- Addition reactions: The double bond is broken, and new atoms or groups are added to the molecule. Examples include the addition of hydrogen (hydrogenation), halogens (halogenation), or water (hydration).
- Polymerization: Alkenes can undergo polymerization, where many alkene molecules join together to form long-chain polymers. This reaction is fundamental to the production of plastics such as polyethylene and polypropylene.
- Oxidation: Alkenes can be oxidized to form alcohols, aldehydes, ketones, or carboxylic acids, depending on the conditions.
Uses of Alkenes
Alkenes are incredibly important in the chemical industry. Ethene, also known as ethylene, is one of the most widely produced chemicals in the world and is used to make polyethylene, the most common plastic. Propene (propylene) is used to produce polypropylene, another important plastic, as well as chemicals like acetone and propylene oxide.
Alkenes also play a role in the production of fuels and synthetic materials. The addition reactions they undergo are essential in creating a variety of valuable products, such as alcohols, plastics, and synthetic rubbers.
What are Alkynes?
Structure and Bonding of Alkynes
Alkynes are hydrocarbons that contain at least one carbon-carbon triple bond. The general formula for alkynes is CnH2n−2C_nH_{2n-2}. Like alkenes, alkynes are more reactive than alkanes due to the presence of multiple bonds between carbon atoms. The triple bond consists of one sigma bond and two pi bonds, which are even weaker than those in alkenes, making alkynes highly susceptible to chemical reactions.
The simplest alkyne is ethyne (C₂H₂), commonly known as acetylene. It consists of two carbon atoms connected by a triple bond, with each carbon bonded to one hydrogen atom. Alkynes can also have branched structures, but due to the rigidity of the triple bond, they are less flexible in their bonding than alkenes.
Physical Properties of Alkynes
Alkynes share similar physical properties with alkenes and alkanes, but their increased reactivity makes them somewhat more chemically active. They are non-polar molecules and are insoluble in water, though they dissolve in non-polar solvents like benzene and ether. As with alkenes, the physical properties of alkynes vary with molecular size. Smaller alkynes, such as ethyne, are gases at room temperature, while larger alkynes are liquids or solids.
Chemical Reactions of Alkynes
Alkynes are highly reactive due to their triple bond, which can undergo a variety of addition reactions. Some of the most important reactions include:
- Hydrogenation: Alkynes can be hydrogenated, meaning they can react with hydrogen gas to form alkenes or alkanes. This process is used in the production of margarine from vegetable oils.
- Halogenation: Alkynes can react with halogens like chlorine or bromine to form dihalides. This reaction is widely used in organic synthesis.
- Hydration: Alkynes can also undergo hydration, where water adds across the triple bond. The resulting product is an alcohol or a ketone, depending on the conditions.
The versatility of alkynes in chemical reactions makes them important in both laboratory research and industrial processes.
Uses of Alkynes
Alkynes, particularly acetylene, have several industrial uses. Acetylene is widely used as a fuel in oxy-acetylene welding and cutting because of its extremely high flame temperature. It is also used in the production of chemicals
such as vinyl chloride, which is used to make PVC (polyvinyl chloride), a common plastic.
Acetylene is also employed in the synthesis of organic compounds, such as in the production of synthetic rubber and various pharmaceuticals.
Conclusion
Alkanes, alkenes, and alkynes represent the three basic types of hydrocarbons, each with its own unique characteristics and chemical reactivity. While alkanes are simple, saturated compounds that form the basis of fuels and lubricants, alkenes and alkynes are unsaturated hydrocarbons with reactive double and triple bonds that make them essential in the production of plastics, synthetic materials, and many important chemicals.
The study of these hydrocarbons opens up a world of possibilities, from the fuels that power our cars to the plastics that shape our daily lives. As we continue to explore their chemical properties and reactions, we unlock new ways to harness their potential for innovation, sustainability, and progress.
By understanding alkanes, alkenes, and alkynes, we gain a deeper appreciation for the molecular world around us, and the powerful forces that drive the chemistry of life.