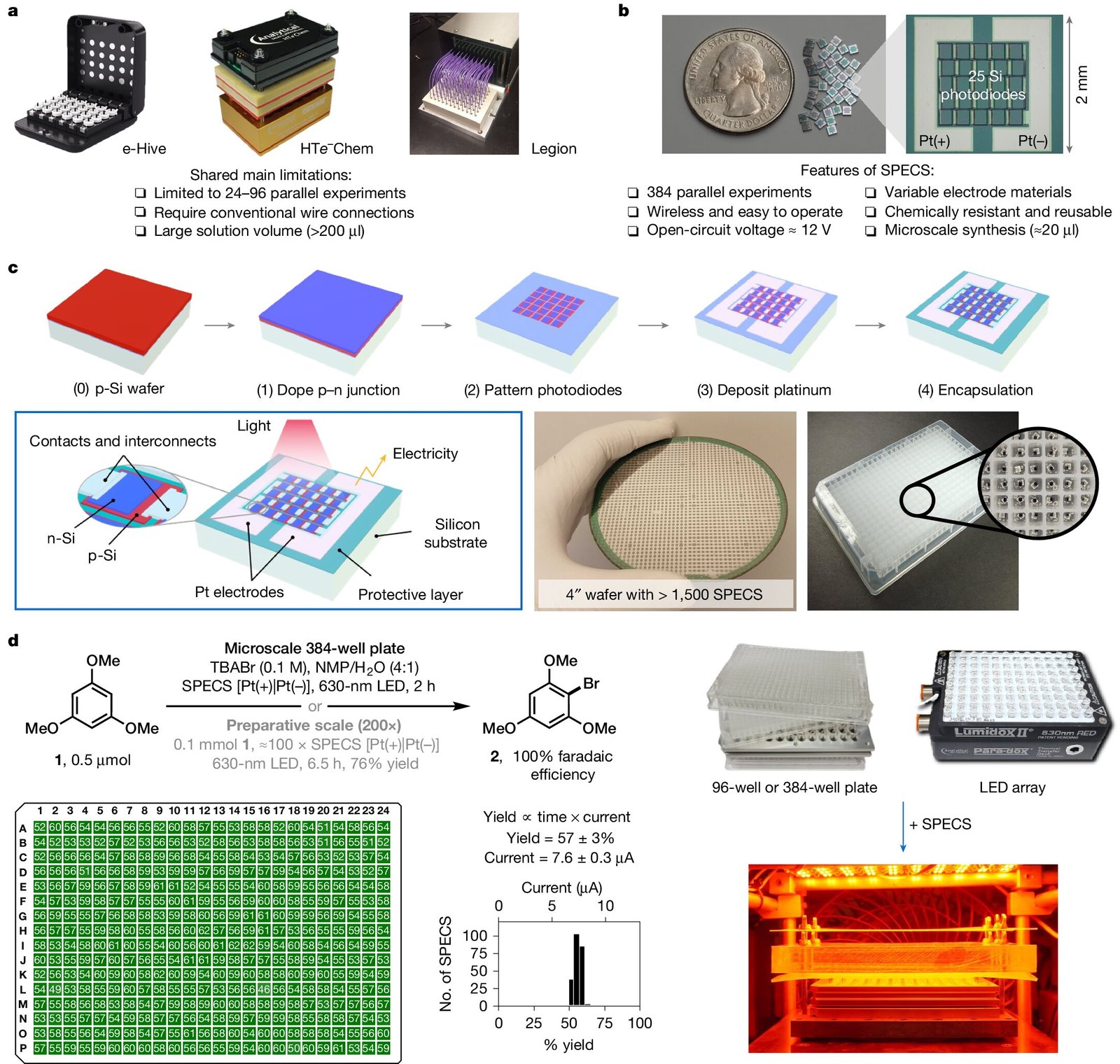
In a groundbreaking achievement, a combined team of chemists and nano-scientists from Cornell University has developed an innovative wireless microelectronic device powered by light that can convert a standard well plate into an array of small electrochemical reactors. This advancement, which holds significant promise for the future of organic chemistry, is described in their recent paper published in Nature. Alongside the paper, Thomas O’Brien and Alastair Lennox from the University of Lausanne offer insights into the work in a “News and Views” commentary within the same issue, highlighting the implications of this technology.
One of the most significant challenges chemists have faced in the field of electrochemistry—particularly in organic reactions—is scaling up experiments in a high-throughput manner. Conducting multiple experiments in parallel typically requires vast quantities of equipment, such as numerous electrodes and wires. For instance, when working with a 384-well plate, 768 electrodes and 768 corresponding wires are needed to connect the wells to a power source. This leads to a cumbersome setup that often limits the number of experiments that can be run simultaneously.
To solve this issue, the research team at Cornell first explored a more compact system of ribbon cables in 2021. These cables allowed them to conduct multiple electrochemical reactions at once—24 reactions in total—while still using traditional wired systems. While this was an improvement, it was far from an ideal solution. The underlying issue—the need for substantial wires and electrodes for each individual experiment—remained unresolved. However, this exploration ultimately inspired a revolutionary idea: what if light could replace electricity as the power source?
The team’s solution to this problem is an unprecedented light-powered reactor device capable of using virtually any size well plate. By harnessing proven solar-cell technology, the device collects light and converts it into electrical power. Crucially, each solar cell in a panel powers a reactor positioned right beneath it, and the team calls these miniature interfaces “small photo-electronics for electrochemical synthesis,” or SPECS. Because each SPECS unit is self-sustained and operates independently, the well plates are able to support unique electrochemical reactions within each individual well. This breakthrough allows for seamless scalability, meaning researchers can now conduct a wide variety of experiments using a single piece of equipment without the complex wiring previously required.
Unlike traditional systems that rely on direct electrical connections to electrodes for energy delivery, this new device uses light to efficiently power each unit. When used in conjunction with a well plate, each well houses its own individual reactor powered by light via solar cells situated on the surface. This setup allows researchers to explore multiple chemical reactions in parallel, all with minimal infrastructure. Whether a lab needs to conduct an experiment with a standard 96-well plate, a 384-well plate, or even larger formats, the device can be adapted to fit the needs of each specific set of reactions. The new device represents a tremendous leap forward in simplifying and accelerating high-throughput experimentation in electrochemistry.
The team tested the new device using a range of known chemical reactions and was able to validate the system’s capabilities. However, their ultimate goal lies not just in making known reactions easier to conduct, but in utilizing this device to explore entirely new chemistry. The light-powered technology promises to revolutionize how electrochemical reactions are conducted, providing chemists with an unprecedented ability to accelerate the pace of their work.
Moreover, this development highlights an exciting frontier in research, one in which electrochemical synthesis—the process of using electrical energy to drive chemical reactions—can be streamlined and made much more efficient. Traditionally, electrochemical reactions play a pivotal role in a wide array of chemical and pharmaceutical industries, as well as in green energy technologies. Having the ability to rapidly conduct and experiment with numerous reactions in parallel without the labor-intensive setup of electrodes, wires, and power sources will significantly speed up research timelines. This innovation could ultimately lead to faster discoveries of novel chemical processes and materials that might otherwise have taken years to bring to light.
As it stands, electrochemical processes are crucial in several vital industries, such as materials science, energy storage, and the pharmaceutical sector. Electrochemical reactions drive the creation of products like batteries, fuel cells, and even synthetic fuels, making any advancement in this area a potential game-changer for sustainability and future technologies. With this new development in mind, the hope is that this wireless, light-powered approach could also find wide application in these sectors, driving efficiency and innovation on a global scale.
The team has already made significant progress, but the real excitement begins as they look forward to pushing the boundaries of electrochemistry even further. The ability to conduct efficient, parallel electrochemical experiments has far-reaching potential across many fields, from synthetic chemistry and material science to the development of clean energy systems. Researchers will now be able to experiment more frequently and with fewer limitations, which could open doors to new breakthroughs in a multitude of applications.
References: Bartosz Górski et al, Light-harvesting microelectronic devices for wireless electrosynthesis, Nature (2025). DOI: 10.1038/s41586-024-08373-1
Thomas M. O’Brien et al, Electrochemical synthesis goes wireless, Nature (2025). DOI: 10.1038/d41586-024-04106-6