In the sleek corridors of the University of Michigan’s materials science labs, something quietly revolutionary has emerged—a material long assumed to be inert and inflexible in the realm of electricity has come to life in an entirely new way. Silicone, the trusted workhorse of waterproof coatings, medical implants, and kitchenware, has now crossed an unexpected threshold: it can conduct electricity.
This transformation shakes a fundamental assumption that has held sway for over a century. Silicones—comprised of silicon, oxygen, and carbon-based side groups—have always been seen as insulators. But now, thanks to a discovery by a research team led by Professor Richard Laine, this familiar family of materials has joined the elite club of semiconductors, ushering in possibilities for flexible electronics, wearables, displays, and smart clothing previously locked behind the barriers of physics and chemistry.
“This material opens up the opportunity for new types of flat-panel displays, flexible photovoltaics, wearable sensors or even clothing that can display different patterns or images,” said Laine, a professor of materials science and macromolecular science at U-M and corresponding author of the study recently published in Macromolecular Rapid Communications.
At first glance, this might seem like an incremental update in polymer chemistry. But beneath the surface lies a rethinking of molecular architecture—a leap that could ripple across sectors as diverse as consumer tech, biomedicine, and energy. Let’s delve into the strange new world where silicone, once only a silent protector against water and heat, now speaks in color and electricity.
The Classical Character of Silicone: Stalwart but Silent
Silicone materials, known scientifically as polysiloxanes and silsesquioxanes, have long been loved for what they don’t do. They don’t conduct electricity. They don’t dissolve in water. They don’t degrade easily. These qualities make them excellent insulators—perfect for biomedical implants, sealants, and electronics coatings. But their stubborn resistance to electricity has also kept them away from the front lines of modern electronic innovation.
This insulating behavior stems from their basic molecular structure. Imagine a zigzag backbone of alternating silicon and oxygen atoms—Si–O–Si—dangling with soft, organic arms. These arms, typically methyl or phenyl groups, help keep the material flexible and stable. The backbone itself forms a semi-open lattice that resists electron flow. At the atomic level, the Si–O bonds are just too bent—about 110 degrees—to allow electrons to hop from one site to the next in the way metals or semiconductors allow.
That’s why silicon—the crystalline cousin of silicone—has long been the star of semiconductors, while silicone stayed behind as a bystander. Until now.
A Serendipitous Discovery in Molecular Engineering
Sometimes, in science, you don’t set out to change the rules—you simply notice that one rule doesn’t seem to apply.
Laine’s team was originally exploring new types of copolymers—long molecules made by combining two distinct types of repeating units. In this case, they were experimenting with hybrids of cage-structured and linear silicone molecules. The goal? To tweak mechanical properties like flexibility, heat resistance, or solubility by adjusting how these components linked together in three-dimensional space.
But something unexpected emerged. As the team analyzed these copolymers, they began noticing behaviors that didn’t align with typical insulating materials. Under certain conditions, the silicone began absorbing light—and even emitting it.
“We stumbled upon a system that started to show signs of semiconductivity,” said Laine. “That was the eureka moment. This wasn’t just a better sealant or a tougher rubber. We were looking at a whole new class of electronic material.”
It was a quiet revolution: a shift in bond angles—imperceptible to the eye but fundamental to physics—had rewritten the rules of electron behavior.
Si–O–Si: A Bond with Untapped Potential
To understand how silicone could become semiconductive, we must enter the realm of electron orbitals and bond angles. In traditional silicone, the Si–O–Si bonds form at roughly 110°, which creates an angular, winding path unsuitable for the continuous motion of electrons. Think of it as trying to roll a marble through a hallway full of turns and dead ends.
But in the new copolymer structures the team discovered, these bond angles opened up—stretching from 110° to 140° in the material’s resting (or ground) state. Even more striking, in an excited state—when electrons jump to a higher energy level—the bonds straighten further to 150°. This near-linear alignment begins to resemble the kind of pathway electrons can use to travel across the material—a kind of molecular autobahn.
“This allows an unexpected interaction between electrons across multiple bonds, including Si–O–Si bonds in these copolymers,” Laine explained. “The longer the chain length, the easier it is for electrons to travel longer distances.”
This structure effectively creates two states—non-conductive and conductive—that define all semiconductors. When the material is in its ground state, it behaves like an insulator. But with the right energy input, such as light or electricity, it flips into a conductive state, enabling electrons to flow.
This discovery marks the first known example of a silicone variant with this kind of tunable semiconductive property. It wasn’t forced into existence with exotic dopants or high-energy inputs. It emerged from thoughtful—but initially unintended—polymer design.
The Science of Color: Electrons, Photons, and Copolymer Chains
The implications of these semiconducting properties go beyond just switching circuits on and off. They also allow silicone to become a medium for color—a property previously unheard of for these materials.
Traditional silicones are colorless or milky-white. Their structure doesn’t absorb or emit much light. But in the newly discovered copolymer, electrons in excited states absorb energy from UV light and then emit it as visible light. The specific color emitted depends on how far the electrons “fall” from the excited state back to the ground state—a process known as fluorescence.
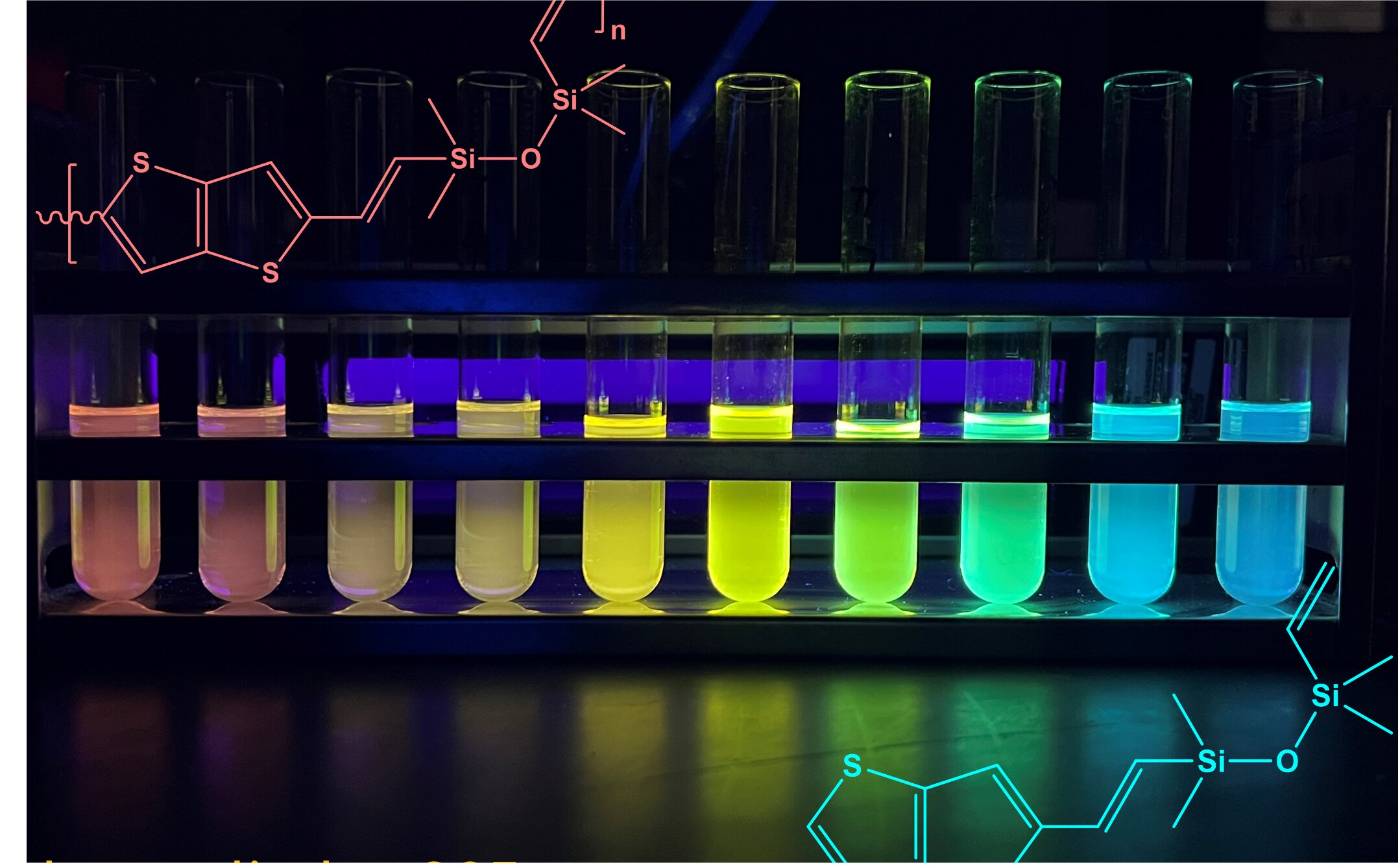
Here’s the twist: that “fall” distance depends on the chain length of the copolymer. Longer chains create smaller energy gaps, resulting in lower energy (redder) light. Shorter chains produce higher energy gaps, which emit bluer light. By controlling the polymer chain length, researchers can essentially tune the material’s color like adjusting a dial.
To demonstrate this effect, Laine’s team separated copolymers of varying lengths into individual test tubes and shined UV light across them. The result was a radiant, fluorescent rainbow—each hue created not by pigments or dyes but by the structure of the material itself.
“We’re taking a material everyone thought was electrically inert and giving it a new life,” said Zijing (Jackie) Zhang, a doctoral student in Laine’s lab and lead author on the paper. “One that could power the next generation of soft, flexible electronics.”
Bending the Future: Applications in Wearables and Displays
So what can you do with silicone that’s flexible, colorful, and electrically active? A lot.
The most obvious applications are in wearable electronics—soft devices that can move with the human body, stretch over curved surfaces, or be embedded directly into clothing. Traditional semiconductors like silicon are rigid and brittle. They crack under strain. But the new silicone copolymer retains its signature softness while gaining new functionality.
Imagine a fitness shirt that changes color based on muscle activity, a bandage that glows to show healing progress, or a flexible display on a water bottle that updates you on hydration levels. Because silicones are already biocompatible and water-resistant, they’re natural fits for these types of applications.
And it doesn’t stop there. The material could be used in lightweight, flexible solar panels that wrap around buildings or cars. Or in display panels that conform to any surface, including the human skin.
This transformation could also lower production costs. Unlike other semiconducting polymers, silicone copolymers can be produced using relatively simple chemistry and scalable processes. They don’t require exotic rare earth elements or high-temperature processing. This positions them well for commercial adoption.
Beyond the Lab: Rethinking Polymer Possibilities
In the broader context of materials science, this discovery offers something even more profound than potential applications—it challenges long-held beliefs about what polymers can do.
For decades, scientists have classified materials into neat categories: metals conduct, insulators resist, semiconductors fall somewhere in between. But now, those boundaries are blurring. A once-inert silicone polymer has not only become semiconductive—it’s tunable, flexible, colorful, and scalable.
“We tend to underestimate the possibilities hidden in materials we think we already understand,” Laine noted. “This discovery shows how much we still have to learn—and how much we can gain—by revisiting the fundamentals with fresh eyes.”
His team is already exploring new variations of the copolymer structure, fine-tuning the balance between cage and linear units, adjusting chain lengths, and investigating how different side groups affect conductivity and color.
Conclusion: A New Chapter in the Story of Silicone
Silicone has long been a silent ally in modern life—a cushion, a seal, a shield. Now it may become something far more dynamic: a storyteller, a sensor, a source of light and information.
What started as a quiet exploration of polymer crosslinking has led to the birth of a new material class—soft semiconductors that marry the familiar virtues of silicone with the transformative power of electronic function.
As we look to a future increasingly defined by flexible technologies—from wearable AI to smart architecture—the discovery of semiconducting silicone may prove to be a pivotal moment. One that reminds us that sometimes, the greatest innovations are not in building new materials, but in seeing the old ones anew.
Reference: Zijing Zhang et al, σ–σ* conjugation Across Si─O─Si Bonds, Macromolecular Rapid Communications (2025). DOI: 10.1002/marc.202500081