Organic chemistry might seem like the domain of lab-coated scientists in distant, high-tech laboratories, but in truth, it’s woven into every part of our daily experience. From the moment we wake up to the minute we fall asleep, organic compounds play a quiet yet vital role in how we live, breathe, clean, cook, communicate, and thrive. They are in our food, our bodies, our clothes, our medicine cabinets—even in the air freshener we spray in the bathroom.
Organic chemistry is the chemistry of carbon-based molecules, and since all life on Earth is carbon-based, it shouldn’t surprise us that these compounds are omnipresent. Some are small and simple, like ethanol in hand sanitizers. Others are vast and complex, like the polymers in plastics or the proteins in our diet. These molecules interact with us constantly, often in invisible but indispensable ways.
Let’s dive into ten of the most common organic compounds you use or encounter every single day, exploring how they work, where they hide, and why your life would be very different without them.
Ethanol: The Versatile Spirit in Your Cabinet and Your Skin
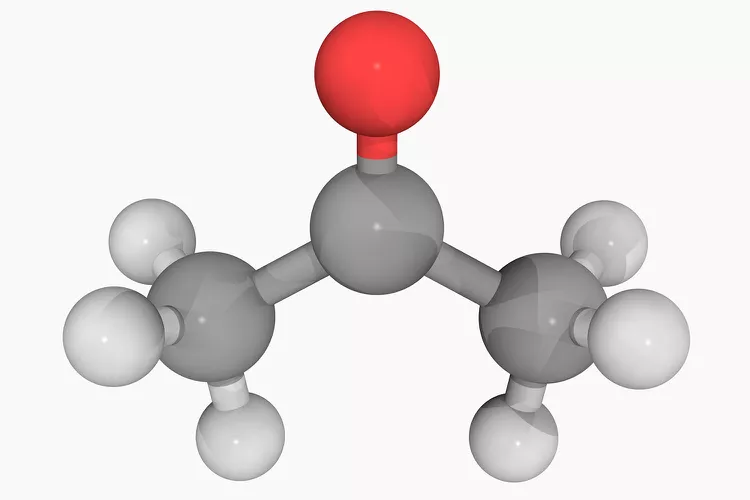
The name “ethanol” might evoke thoughts of alcoholic beverages, and rightly so—it’s the active compound in beer, wine, and spirits. But ethanol is far more than just an intoxicating liquid. It’s one of the most ubiquitous organic molecules in the modern world.
Chemically, ethanol is a simple molecule with just two carbon atoms: CH₃CH₂OH. That “OH” at the end is what classifies it as an alcohol (not to be confused with “alcoholic drinks”). This same group gives ethanol many of its characteristic properties—it’s a solvent, it’s volatile, it kills microbes, and it mixes well with water and oils alike.
In your daily life, ethanol appears in several forms. Aside from beverages, it’s a core ingredient in hand sanitizers, where its antimicrobial properties come in handy—especially in a post-pandemic world. When you rub sanitizer on your hands, it’s the ethanol (or isopropanol) doing the germ-killing work, not the gooey gel that makes it easier to apply.
Ethanol also shows up in cleaning products, cosmetics, perfumes, and even fuels. “Gasohol,” a blend of gasoline and ethanol, is used to power vehicles in many countries. In perfumes and body sprays, ethanol acts as a carrier for the aromatic compounds, helping them evaporate quickly on your skin.
Its volatility makes ethanol useful in cooking too—particularly in flambéing, where a dish is briefly set on fire. So whether you’re cleaning your hands, fueling your car, or sipping wine with dinner, ethanol is never far from the scene.
Acetic Acid: The Sour Spark Behind Vinegar and More
Open a bottle of vinegar, and you’ll immediately encounter the sharp, nose-wrinkling tang of acetic acid. Though it smells aggressive, acetic acid is an essential player in the kitchen and the lab, and it has an important role in biology as well.
Acetic acid, with the formula CH₃COOH, is the simplest carboxylic acid after formic acid. That carboxylic group—COOH—is key to many biochemical reactions. In vinegar, acetic acid is diluted (usually around 5%), giving it just the right amount of pungency for cooking and preserving food. It adds brightness to dressings and sauces and enhances flavors by interacting with both taste receptors and food molecules.
Acetic acid is also a mild disinfectant, often used in natural or eco-friendly cleaning solutions. It cuts through grease, dissolves mineral deposits, and can kill certain bacteria. Many households keep a spray bottle of diluted vinegar on hand for cleaning glass or deodorizing kitchen counters.
Biochemically, acetic acid is part of a much larger story: it’s central to cellular respiration. Inside the mitochondria of your cells, carbohydrates are broken down into acetic acid derivatives before entering the citric acid cycle. So even though you associate it with your salad dressing, acetic acid is also part of the invisible machinery of life inside you.
Citric Acid: The Tangy Power of Citrus and Soft Drinks
The zing of a lemon, the refreshing tartness of a lime, the sour edge of a soda—all of these sensations trace back to one molecule: citric acid. This tricarboxylic acid, with the formula C₆H₈O₇, occurs naturally in citrus fruits and plays a starring role in both food science and human biology.
Citric acid is used in countless products you probably don’t even notice. It’s added to soft drinks, candies, and canned foods to provide tartness and preserve freshness. Its sharp taste is a key flavor component in sour candies and powdered drink mixes. Citric acid also binds to metals like calcium and magnesium, helping soften water and stabilize products. That’s why it’s often found in shampoos, detergents, and soaps.
In your body, citric acid is far more than a tastebud stimulant. It’s a key molecule in the citric acid cycle (also known as the Krebs cycle), which is how your cells generate energy. Every breath you take helps convert nutrients into citric acid, which then gets broken down to produce ATP—the chemical fuel of life.
Thus, whether you’re drinking a lemon soda or running a marathon, citric acid is with you every step of the way.
Glucose: The Sweet Fuel of Life
If there’s a celebrity among organic compounds, glucose deserves the spotlight. It’s the most fundamental sugar in biology, the molecule that powers everything from thought to muscle movement.
With the formula C₆H₁₂O₆, glucose is a simple sugar (monosaccharide) that serves as the primary energy source for cells in nearly all living organisms. It’s found in fruits, vegetables, bread, pasta, and virtually any food containing carbohydrates. When you eat, enzymes break down complex carbohydrates into glucose, which is then absorbed into your bloodstream and transported to cells.
Glucose plays a central role in metabolism. Once inside cells, it undergoes glycolysis, a ten-step process that releases energy. In the presence of oxygen, this process continues in the mitochondria through the citric acid cycle and oxidative phosphorylation, yielding large amounts of ATP.
Outside your body, glucose appears in many consumer products. It’s used in syrups, candies, and energy drinks for a quick sugar boost. In baking, it contributes to browning reactions that give bread its golden crust. In fermentation, yeast consumes glucose to produce alcohol and carbon dioxide—making beer fizzy and bread rise.
Whether you’re sipping orange juice or sprinting across a soccer field, glucose is at the heart of it all.
Sucrose: The Household Sweetener
If glucose is the body’s energy staple, sucrose is the sugar we know best. Common table sugar, derived from sugarcane or sugar beets, is chemically a disaccharide—made of one glucose molecule and one fructose molecule bonded together.
The molecular formula for sucrose is C₁₂H₂₂O₁₁, and it dissolves easily in water, making it ideal for cooking and baking. From cookies and cakes to sauces and syrups, sucrose is ubiquitous in the kitchen. It not only sweetens but also affects texture, shelf life, and browning reactions.
Biologically, sucrose is broken down by enzymes in the small intestine into its component sugars. These are then absorbed and used for energy or stored as fat if consumed in excess.
Sucrose also plays roles in preserving foods (as in jams and jellies), feeding fermenting yeast (in brewing), and even in cosmetics, where it’s used as a natural exfoliant. While it gets a bad rap in nutrition circles when overused, in moderation, it’s a vital and historic part of the human diet.
Caffeine: The Chemical Kick in Your Coffee
That morning ritual of coffee, tea, or energy drink? The boost you feel is powered by caffeine, an organic compound with the formula C₈H₁₀N₄O₂. It belongs to a class of compounds called alkaloids—naturally occurring organic molecules that often have physiological effects.
Caffeine works by blocking adenosine receptors in the brain. Adenosine is a neurotransmitter that promotes relaxation and sleepiness. When caffeine blocks these receptors, you feel more awake and alert.
Caffeine is found naturally in coffee beans, tea leaves, cacao pods, and guarana berries. It’s added to soft drinks, energy drinks, and over-the-counter medications like pain relievers and weight-loss aids.
Its effects extend beyond alertness. Caffeine can enhance memory, increase endurance, and even improve mood—though these effects vary by individual. It’s also a diuretic and a mild stimulant of the central nervous system.
Despite its addictive qualities and occasional side effects, caffeine remains one of the most consumed psychoactive substances on Earth. If you’ve ever faced a deadline, fought off sleep on a road trip, or simply enjoyed a hot morning cup, you’ve experienced the quiet chemistry of caffeine.
Acetylsalicylic Acid: The Molecule Behind Aspirin
Headache? Fever? Minor aches and pains? One of the most familiar solutions is a tiny white tablet of aspirin. The active ingredient is acetylsalicylic acid, an organic compound with the formula C₉H₈O₄.
Aspirin is a derivative of salicylic acid, which comes from willow bark. Chemists modified it by adding an acetyl group to reduce the harshness on the stomach lining. This modification transformed it into one of the most widely used medications in the world.
Acetylsalicylic acid works by inhibiting enzymes called cyclooxygenases (COX), which are involved in the production of prostaglandins—compounds that mediate inflammation, pain, and fever. By reducing prostaglandin synthesis, aspirin offers relief from pain and swelling.
Beyond its role as a painkiller, aspirin is also used in low doses to reduce the risk of heart attacks and strokes. It helps prevent blood clots by thinning the blood—another effect tied to its inhibition of COX enzymes in platelets.
Aspirin was one of the first drugs to be mass-produced and remains a cornerstone of medicine cabinets around the globe. It’s a prime example of how an organic molecule, once hidden in a tree bark, can change human health forever.
Lactic Acid: The Burn in Your Muscles and Yogurt
Anyone who has pushed through a hard workout knows the familiar burn in the muscles. That sensation, once thought to be caused by lactic acid, is part of a complex physiological process. But lactic acid, with the formula C₃H₆O₃, still plays a critical role in both exercise and everyday food.
During intense exercise, when oxygen levels are low, cells switch to anaerobic metabolism. Glucose is broken down into pyruvate, and when oxygen isn’t sufficient for full aerobic respiration, pyruvate is converted into lactic acid. This allows energy production to continue—albeit less efficiently—so you can keep moving.
In the culinary world, lactic acid is a product of fermentation. Bacteria such as Lactobacillus convert sugars into lactic acid, which gives yogurt, sourdough, and kimchi their tangy flavor. It also acts as a preservative by lowering pH and inhibiting spoilage microbes.
Your body also uses lactic acid in normal metabolism. It circulates in your blood, shuttled to the liver where it’s recycled into glucose—a process called the Cori cycle.
Lactic acid bridges biology and culture, showing up in sweat-drenched workouts and in the rich traditions of fermented foods.
Nicotine: The Stimulant in Tobacco
Found in tobacco plants, nicotine is a naturally occurring alkaloid with the formula C₁₀H₁₄N₂. It’s infamous for its addictive properties and its central role in smoking-related health problems, but its chemistry is nonetheless fascinating.
Nicotine acts as a stimulant by binding to nicotinic acetylcholine receptors in the brain, mimicking the neurotransmitter acetylcholine. This interaction leads to increased release of dopamine and other neurotransmitters, resulting in pleasure, alertness, and reduced anxiety—at least temporarily.
Though its primary association is with cigarettes, nicotine is also found in smaller amounts in other members of the nightshade family, like tomatoes, potatoes, and eggplants.
It’s also used in controlled therapeutic settings. Nicotine patches, gum, and lozenges help people quit smoking by providing a regulated dose to reduce withdrawal symptoms.
Despite its addictive potential and health risks, nicotine remains a compound of enormous social and psychological impact—one whose story continues to evolve with changing health trends and regulatory landscapes.
Cellulose: The Organic Scaffold of Plants and Products
The final organic compound on our daily journey is one you encounter constantly—but never taste or smell. It’s cellulose, a long-chain polysaccharide composed of glucose units, and it’s the structural backbone of the plant world.
Cellulose, with the formula (C₆H₁₀O₅)n, is the most abundant organic compound on Earth. It forms the cell walls of plants, giving them strength and rigidity. You encounter it in paper, cotton, cardboard, and even some dietary supplements labeled as “fiber.”
Your body can’t digest cellulose, but that’s not a drawback—it’s a feature. As dietary fiber, cellulose helps regulate digestion, prevents constipation, and supports gut health.
Beyond food and paper, cellulose is used industrially to make biodegradable plastics, insulation materials, and clothing. Modified cellulose derivatives, such as cellulose acetate, are used in photographic films and even eyeglass frames.
Even if you’ve never heard its name, cellulose is all around you—in your jeans, your books, your breakfast cereal, and your backyard trees. It’s the unsung hero of organic chemistry, quietly supporting life from the roots up.