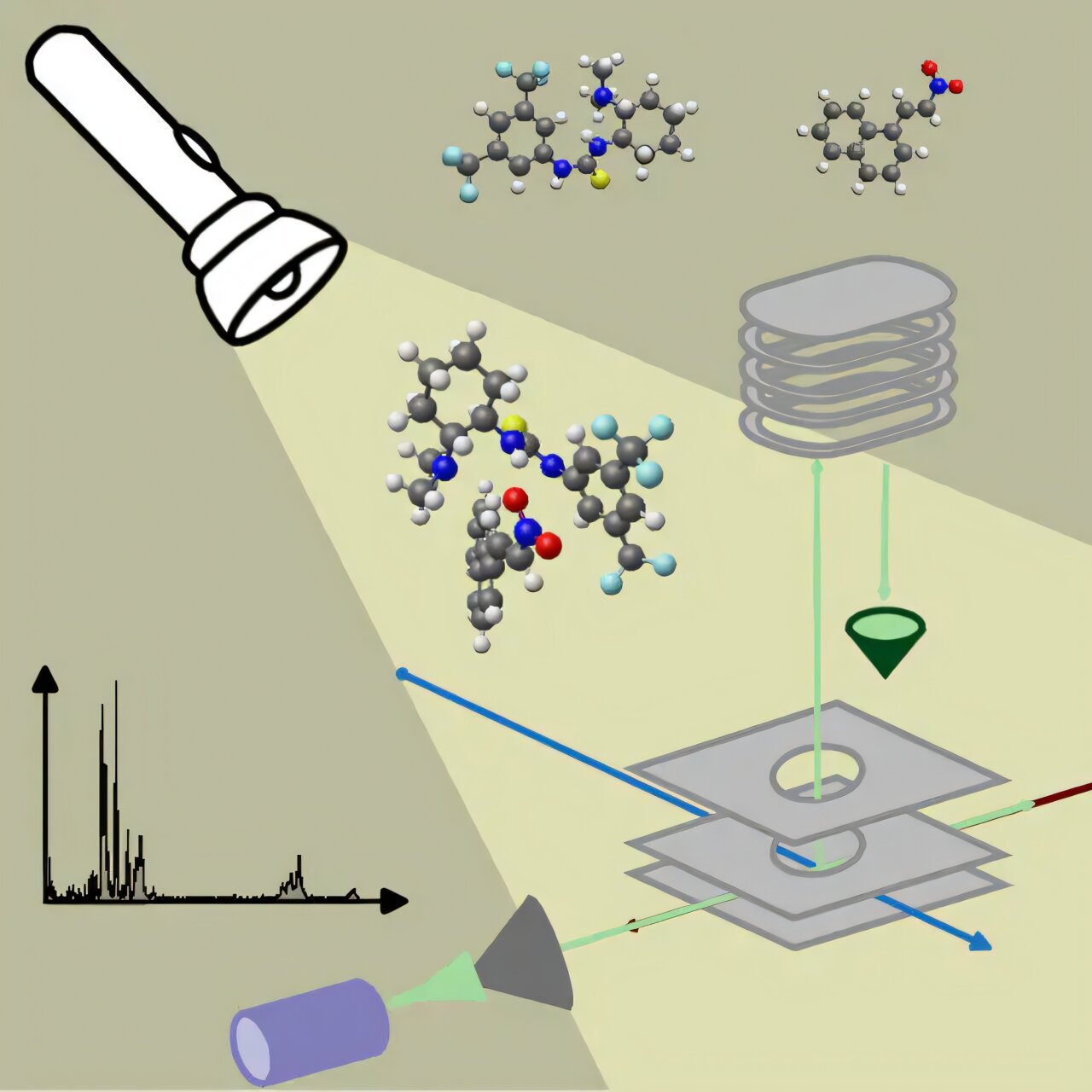
In the invisible theater of molecules, reactions unfold in a chaotic blur, intermediates appearing and vanishing in mere picoseconds. Scientists have long dreamed of freezing that moment—to glimpse how catalysts actually work as they guide molecules along new paths. Now, a pioneering collaboration between researchers at the University of Amsterdam and the HFML-FELIX institute in Nijmegen has done just that.
In a remarkable breakthrough, detailed in a new study published in The Journal of Physical Chemistry Letters, the team has managed to capture, characterize, and analyze a fleeting catalyst-reactant complex in astonishing detail—offering the clearest picture yet of how a powerful class of metal-free catalysts, called organocatalysts, perform their molecular magic.
This is more than just a technical achievement. It’s a leap forward in green chemistry, with implications for safer pharmaceuticals, cleaner industrial processes, and the intelligent design of the next generation of synthetic tools.
Seeing the Invisible: A New Frontier in Catalyst Research
Catalysts are the quiet workhorses of chemistry, enabling reactions that would otherwise be too slow or too inefficient. But while metal-based catalysts have long dominated the field, they come with a price—literally and environmentally. Many are expensive, toxic, or both.
Organocatalysts, by contrast, are small, metal-free molecules that offer a more sustainable and potentially safer alternative. Their ability to control the precise shape and structure—what chemists call the “chirality”—of reaction products makes them invaluable for applications ranging from drug development to materials science.
Still, for all their promise, organocatalysts have kept many of their secrets hidden. Until now, scientists lacked the tools to observe them in action—especially the complex intermediate steps where the real molecular decision-making takes place.
But that’s exactly what the Amsterdam-Nijmegen team has managed to uncover.
Lasers, Molecular Beams, and Frozen Reactions
The team, led by Prof. Wybren Jan Buma of the University of Amsterdam’s Molecular Photonics group, turned to one of Europe’s most powerful scientific instruments: the FELIX free-electron laser, operated by the Dutch Research Council NWO at the HFML-FELIX institute in Nijmegen.
FELIX is not your ordinary lab laser. With the ability to generate intense, tunable pulses of infrared light across a broad spectral range (from 650 to 3500 cm⁻¹), it can reveal the precise “vibrational fingerprints” of molecules—showing how atoms are connected and how they move.
In the experiment, the team merged a cold molecular beam—containing Takemoto’s catalyst and its reactant—with FELIX’s infrared laser. This allowed them to isolate and study a fleeting intermediate: the moment when the catalyst and reactant are bound together in preparation for transformation.
The vibrational data revealed key shifts in the catalyst’s structure. Hydrogen bonds formed. Atoms twisted into new alignments. Entire sections of the molecule flexed into place to accommodate the guest. The result? A molecule transformed, ready to shepherd the reaction forward.
But perhaps most impressively, the team managed to do this with neutral species—uncharged molecules that are notoriously difficult to detect mid-reaction. Prior studies had mostly focused on ions, which are easier to trap and study. This work breaks that barrier.
Quantum Chemistry Meets Experimental Precision
Of course, the laser data alone isn’t enough. The researchers also used advanced quantum chemical calculations to interpret the vibrational spectra, mapping the observed molecular fingerprints onto specific geometric structures.
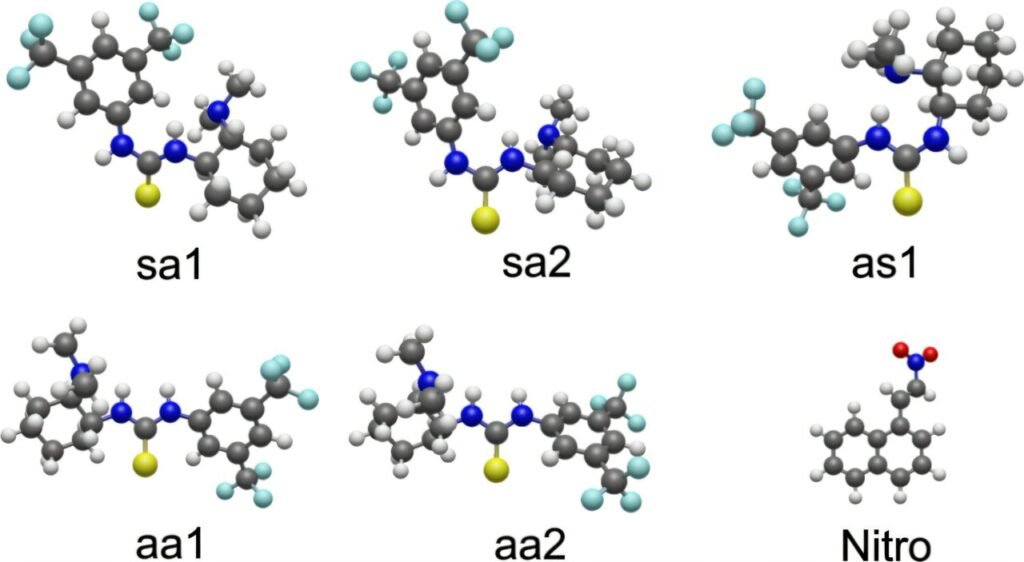
Together, the experimental and theoretical tools allowed the team to unambiguously determine how the catalyst morphs during its interaction with the reactant—a feat that had remained out of reach for decades.
And it’s not just a snapshot. By analyzing subtle changes in energy, motion, and bond strength, the study uncovers the underlying dynamics that govern the catalytic process. It shows, for instance, how the catalyst temporarily adopts a higher-energy shape—one it wouldn’t otherwise maintain—just to make the reaction happen.
“That intermediate is like a moment of molecular tension,” said one researcher familiar with the project. “It’s a state of strain and purpose—a necessary pause before transformation.”
By “freezing out” this moment under high-collision conditions in the molecular beam, the team could trap the intermediate in time, allowing its story to be told.
Implications for Green Chemistry and Rational Design
So what does this mean for the world beyond the lab?
For one, it provides a critical piece of the puzzle for rational catalyst design. Instead of relying on trial and error, chemists can now visualize how specific structures and functional groups interact in real time. This makes it possible to design organocatalysts that are not only more efficient but also more selective—delivering the exact molecular product needed, every time.
That’s a game-changer for pharmaceuticals, where the difference between one molecular enantiomer and another can mean the difference between a life-saving drug and a harmful side effect.
Moreover, the methodology is broadly applicable. The techniques demonstrated here can be used to study other organocatalysts, and even metallo-catalyzed reactions that have long resisted structural analysis. The ability to access and characterize reactive intermediates—once thought too elusive to touch—opens a new era in catalytic chemistry.
“This study proves that the curtain can be pulled back on even the most fleeting of molecular moments,” said Buma. “And when you can see clearly, you can build better.”
From the Lab to a Cleaner Tomorrow
At its core, this research is about precision. About catching the molecular players in the act. About using the brightest tools of physics, chemistry, and computation to illuminate what was once only inferred.
But it’s also about responsibility.
As the world seeks greener, safer, and more efficient chemical solutions, understanding how reactions really work—from beginning to end—has never been more important. This study brings us one step closer to that goal, proving that sustainability and scientific sophistication can go hand in hand.
From lasers and molecular beams to the quantum world of vibrating atoms, this is chemistry in high definition. And in that clarity lies the promise of a cleaner, smarter future.
Reference: Piero Ferrari et al, Light on Catalytic Reaction Mechanisms: Uncovering the Conformation of Thiourea-Based Organocatalysts and Their Interaction with Nitroolefins Using Mid-infrared Spectroscopy, The Journal of Physical Chemistry Letters (2025). DOI: 10.1021/acs.jpclett.5c01093