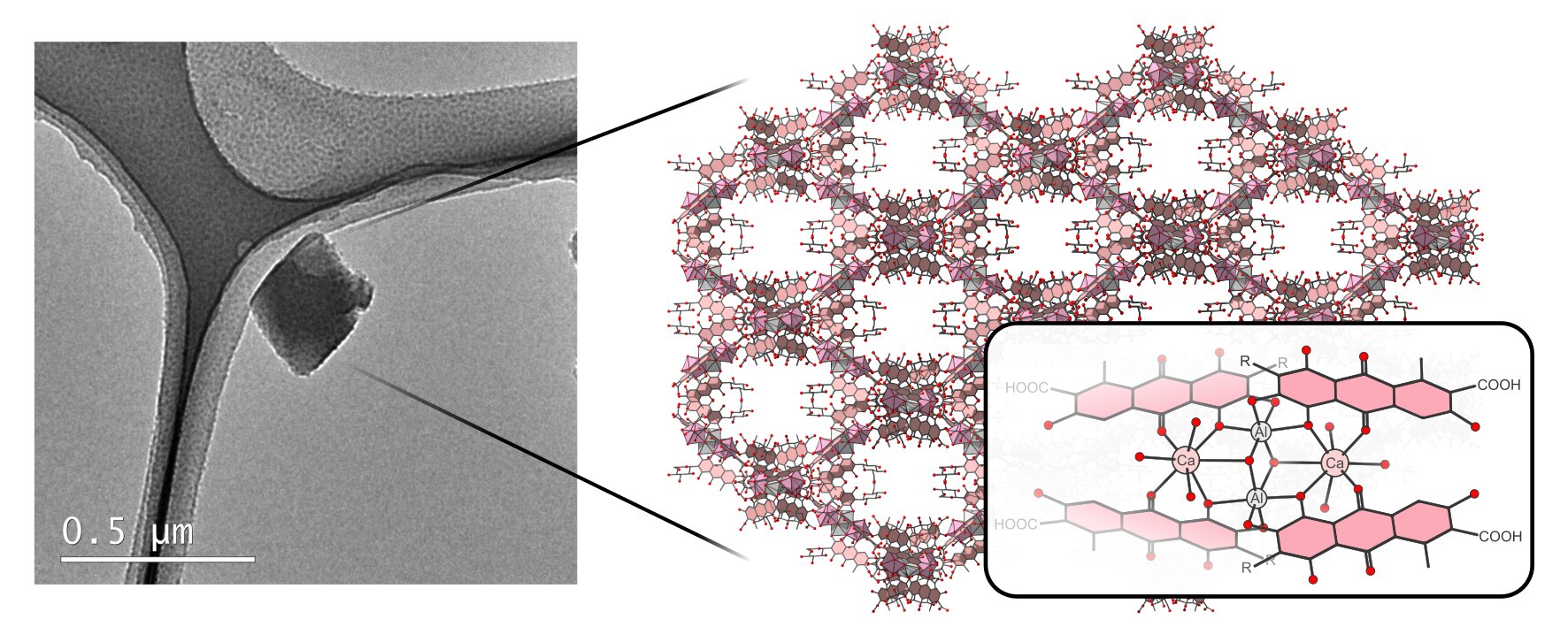
Across ancient manuscripts, medieval frescoes, baroque oil paintings, and even the pink coating of modern candy, a vivid red pigment has silently threaded its way through human history. Carmine, derived from the dried and crushed bodies of the cochineal insect, has painted empires and sweetened treats for centuries. But despite its ubiquitous use and long-standing cultural significance, carmine has kept a curious secret—one that has only now been uncovered by cutting-edge science. Using a revolutionary technique in electron crystallography, researchers at Stockholm University have peered into the atomic heart of this iconic pigment and discovered a hidden, porous architecture of striking complexity.
The revelation is as stunning as the pigment’s hue. Carmine, once thought to be chemically straightforward, is in fact a crystalline structure of intricate beauty—a metal-organic network woven together by aluminum, calcium, and carminic acid. The findings, published in the journal Crystal Growth & Design, are transforming not just our understanding of one of history’s most famous colors, but also opening the door to new applications in environmental science and material chemistry.
This is the story of how a 16th-century dye has become a 21st-century nanomaterial.
The Insect Behind the Hue
The tale of carmine begins in Central and South America, where Indigenous peoples, particularly the Aztecs and the Maya, first harvested the scale insect Dactylopius coccus from prickly pear cacti. The cochineal, when crushed, yields carminic acid—a bright red anthraquinone derivative that forms the basis of the pigment. Spanish conquistadors were dazzled by the richness of the color and quickly monopolized its production. Carmine became one of the most valuable exports of the Spanish Empire, second only to silver. Painters, textile manufacturers, and royalty across Europe clamored for the pigment, entranced by its permanence and brilliance.
Centuries later, even in a world flooded with synthetic dyes, carmine endures. It continues to color candies, yogurts, lipsticks, and artists’ paints—thanks in part to the growing demand for natural ingredients. But despite its long and global usage, one essential detail had remained elusive: the true atomic structure of carmine.
The Puzzle of Pigment Crystallography
For decades, scientists knew that carmine was crystalline, but its structure resisted full characterization. The problem wasn’t theoretical—it was physical. Traditional techniques like single-crystal X-ray diffraction require large, high-quality crystals to determine molecular arrangements with precision. Carmine crystals, however, are notoriously small and delicate, more akin to microscopic shavings than robust gemstones. This made it nearly impossible to probe their internal architecture using standard tools.
Enter MicroED: a revolutionary technique in electron crystallography. Short for Micro Electron Diffraction, MicroED allows researchers to analyze minuscule crystalline materials—down to the nanometer scale—by firing electrons at them rather than X-rays. Because electrons interact more strongly with matter than X-rays, they produce more detailed images of small structures. The technique has opened up entirely new domains of structural biology and materials science.
At Stockholm University, Erik Svensson Grape, then a Ph.D. student in the Department of Chemistry, saw an opportunity. Curious about the molecular world and its secret geometries, he chose carmine as a test subject—not because anyone expected to find something new, but because it had been overlooked. “It was truly surprising that such a long-used pigment made from a naturally occurring molecule had this type of structure,” Grape says. What began as a curiosity project quickly morphed into a scientific revelation.
A Molecular Kaleidoscope
What Grape and his colleagues found using MicroED was astonishing. Carmine is not just a simple mixture of components. It is a metal-organic complex with a precisely arranged, three-dimensional porous architecture.
At its core, the structure comprises two aluminum ions, two calcium ions, and four molecules of carminic acid. These units organize themselves into a repeating crystalline lattice, forming cavities and channels that resemble the framework of modern nanoporous materials. It is, in effect, a naturally occurring example of what chemists now refer to as a metal-organic framework (MOF)—a class of materials famous for their sponge-like ability to trap and interact with other molecules.
Chemists have only recently begun to design and synthesize MOFs for specific applications such as catalysis, drug delivery, carbon capture, and hydrogen storage. That a pigment like carmine—a substance used since the 1500s—should already embody such an advanced structural concept was an exhilarating surprise. “The first commercially available nanoporous materials were only developed in the middle of the 20th century,” Grape notes. “And yet here was carmine, built by nature and shaped by ancient artisans, operating on the same principles.”
Porosity with a Purpose
The implications of carmine’s structure extend far beyond academic curiosity. Porous materials have unique properties: they can adsorb gases, hold ions, separate chemicals, and serve as platforms for catalysis. Carmine’s molecular architecture suggests that it might possess many of these properties—only hidden in plain sight until now.
One immediate application is in cultural heritage science. The well-defined crystal structure of carmine could help art conservators and archaeologists identify and authenticate historical pigments in ancient artworks, manuscripts, and textiles. Because the pigment has a unique structural “fingerprint,” advanced imaging techniques could now be used to trace its presence in places where visual inspection alone would fail.
More futuristic possibilities lie in environmental science. Porous materials are powerful adsorbents: substances that can concentrate and trap molecules on their surfaces. An adsorbent like carmine could, for example, be engineered to capture pollutants from water or air. This aligns with an urgent global need to develop sustainable methods for environmental remediation. With further research, carmine’s natural framework might be harnessed or even modified to function in wastewater treatment or gas filtration.
Grape’s team is also considering whether the pigment’s porous structure could find applications in energy storage or chemical sensors. While such uses are still speculative, the precedent set by other MOFs suggests a fertile ground for innovation.
A Natural Answer to Synthetic Dilemmas
Beyond its structural surprises, carmine’s reemergence as a natural nanomaterial comes at a pivotal moment. In recent years, synthetic food dyes and artificial coloring agents have faced increasing public scrutiny. Concerns about potential health risks, allergic reactions, and links to hyperactivity in children have led to stricter regulations and consumer pushback.
In contrast, natural pigments like carmine—despite their entomological origins—are seen as safer, more transparent alternatives. However, concerns about sustainability, allergenicity (some people are sensitive to cochineal extract), and animal ethics have also sparked debate. Understanding the exact structure of carmine can help address some of these issues. It allows manufacturers to better control its purity, develop derivatives with fewer allergens, or even explore synthetic biology routes to replicate its structure without the insect.

This fusion of ancient tradition and modern science presents an appealing alternative to industrial colorants. As Grape notes, “Natural pigments like carmine may soon be used more often in the food industry,” especially as clean labeling and natural sourcing become selling points for consumers.
The Rediscovery of Ancient Innovation
Carmine’s newfound structure is a humbling reminder of how much remains hidden in the materials we think we know. It is a paradox of modern science that sometimes the oldest substances, passed down through generations, still guard the most profound secrets. That a humble red pigment could be a model for nanotechnology, a tool for conservation, and a possible environmental workhorse speaks volumes about nature’s ingenuity—and our evolving capacity to understand it.
Moreover, this discovery is not just about electrons and atoms. It’s about continuity across time. From the Aztecs grinding insects under stone to 21st-century scientists blasting crystals with electrons, carmine represents a thread that binds art, chemistry, history, and technology. In uncovering its hidden order, we are not just refining science—we are rediscovering the brilliance of human curiosity across centuries.
The Road Ahead: Designing With Nature in Mind
While the structure of carmine has now been mapped, its full capabilities have only begun to be explored. Future research could involve testing the pigment’s performance under various environmental conditions, mapping its adsorption capabilities with specific pollutants, or attempting to re-engineer its framework for more targeted applications.
Scientists may also seek to synthesize analogs of carmine—materials that replicate its architecture but use different metals or ligands to fine-tune performance. This biomimetic approach, inspired by a molecule hidden inside paint and candy, could inform the design of new porous materials that are both sustainable and efficient.
The tools that enabled this breakthrough—namely MicroED—will also continue to evolve. As they become more widespread, other mysterious or poorly understood materials from nature may finally yield their secrets. Carmine may be only the first in a long line of rediscovered natural materials that turn out to be more sophisticated than we ever imagined.
A Legacy Written in Red
In the end, carmine’s story is a testament to the enduring value of curiosity. What began as a simple investigation into the makeup of a familiar pigment has become a case study in the unexpected richness of the natural world. The pigment that once signaled wealth in Renaissance portraits and now brightens the shelves of grocery stores has revealed itself to be not just a color, but a crystalline marvel.
Its story is far from over. As science continues to peel back the layers of the materials that color our world—literally and figuratively—carmine stands as a vivid symbol of what we still have to learn. In its microscopic pores lie the pathways to cleaner water, smarter sensors, and safer dyes. In its shimmering red, a story that stretches from Incan textiles to nanotechnology.
And as researchers like Grape and his team have shown, sometimes all it takes to make a world-changing discovery is to look again—more closely this time—at something we thought we already knew.
Reference: Erik Svensson Grape et al, Brilliantly Red: The Structure of Carmine, Crystal Growth & Design (2025). DOI: 10.1021/acs.cgd.5c00185