In the sprawling world of chemistry, few compounds have made as vast an impact on human civilization as urea. With its unassuming molecular formula—CO(NH₂)₂—urea is at the heart of modern agriculture, essential for feeding billions by enriching soils with nitrogen. But behind this critical compound lies a hidden energy burden, one that scientists have long grappled with. Now, thanks to pioneering research from a team at Sun Yat-Sen University, a radical shift in how we make urea might be just around the corner.
Why Urea Matters
First isolated from urine by Dutch chemist Herman Boerhaave in the 18th century, urea became historically significant when German chemist Friedrich Wöhler synthesized it from an inorganic compound in 1828, breaking the long-held belief that organic compounds could only be produced by living organisms. This discovery didn’t just birth organic chemistry—it revolutionized our understanding of life itself.
Today, urea is primarily used as a nitrogen-based fertilizer, enabling crops to grow more efficiently and abundantly. It’s also employed in animal feed, plastics, pharmaceuticals, and even diesel exhaust systems to reduce harmful emissions. Global demand for urea is staggering, with over 180 million metric tons produced annually. And yet, this vital compound comes at a steep environmental cost.
The Traditional Path: Effective, but Energy-Hungry
The standard method for producing urea begins with ammonia, which itself is created by the Haber-Bosch process—a reaction between nitrogen (N₂) from air and hydrogen, often derived from natural gas. The process requires high temperatures (400–500°C) and pressures (150–200 atm), making it one of the most energy-intensive operations in the chemical industry.
Once ammonia is synthesized, it reacts with carbon dioxide (CO₂) in a two-step sequence. First, the two gases combine to form ammonium carbamate under pressure and heat. Then, this intermediate compound is decomposed into urea and water at lower pressure.
Despite its effectiveness, this method consumes approximately 1% of the world’s energy supply. The carbon footprint is also substantial—an ironic twist, considering that CO₂ is used in the reaction. With climate change concerns escalating and energy costs rising, scientists are racing to reimagine urea production for a more sustainable future.
A Radical Proposal: Making Urea Without Ammonia
Imagine skipping the ammonia step entirely. Instead of reacting ammonia with CO₂, what if we could make urea directly from nitrogen and carbon dioxide—the very molecules floating freely in air and industrial waste gases? It’s a bold idea, but one that holds tremendous promise.
To achieve this, researchers have turned to a technique called electrosynthesis. Using electrolyzers—devices that apply electricity to drive chemical reactions—scientists aim to reduce nitrogen and carbon dioxide simultaneously in a single system. Theoretically, this could enable a direct route to urea while slashing energy usage and emissions.
But in practice, it’s proven devilishly difficult. The problem? Side reactions. Nitrogen, a notoriously inert molecule, often gets fully hydrogenated to form ammonia, while carbon dioxide tends to produce carbon monoxide or formic acid. These byproducts ruin efficiency and clog up purity. For years, researchers have searched for a way to coax these stubborn molecules into joining forces to form urea—without veering off course.
A Breakthrough at Sun Yat-Sen University
In a recent landmark study published in Nature Nanotechnology, scientists at Sun Yat-Sen University in China have unveiled an ingenious solution: a proton-limited environment created using a porous solid-state electrolyte. This setup allows for the direct electrosynthesis of pure urea—not from refined ammonia, but from pretreated flue gas, a waste product of combustion.
Led by researchers Yan-Chen Liu and Jia-Run Huang, the team constructed a novel electrolyzer that operates without liquid electrolytes, using instead a porous solid matrix that restricts the availability of protons. This limitation is crucial. By suppressing the hydrogen evolution reaction—which normally competes with nitrogen and carbon dioxide reduction—the system encourages the formation of urea rather than ammonia or hydrogen gas.
Chemistry in Harmony: The Key to C–N Coupling
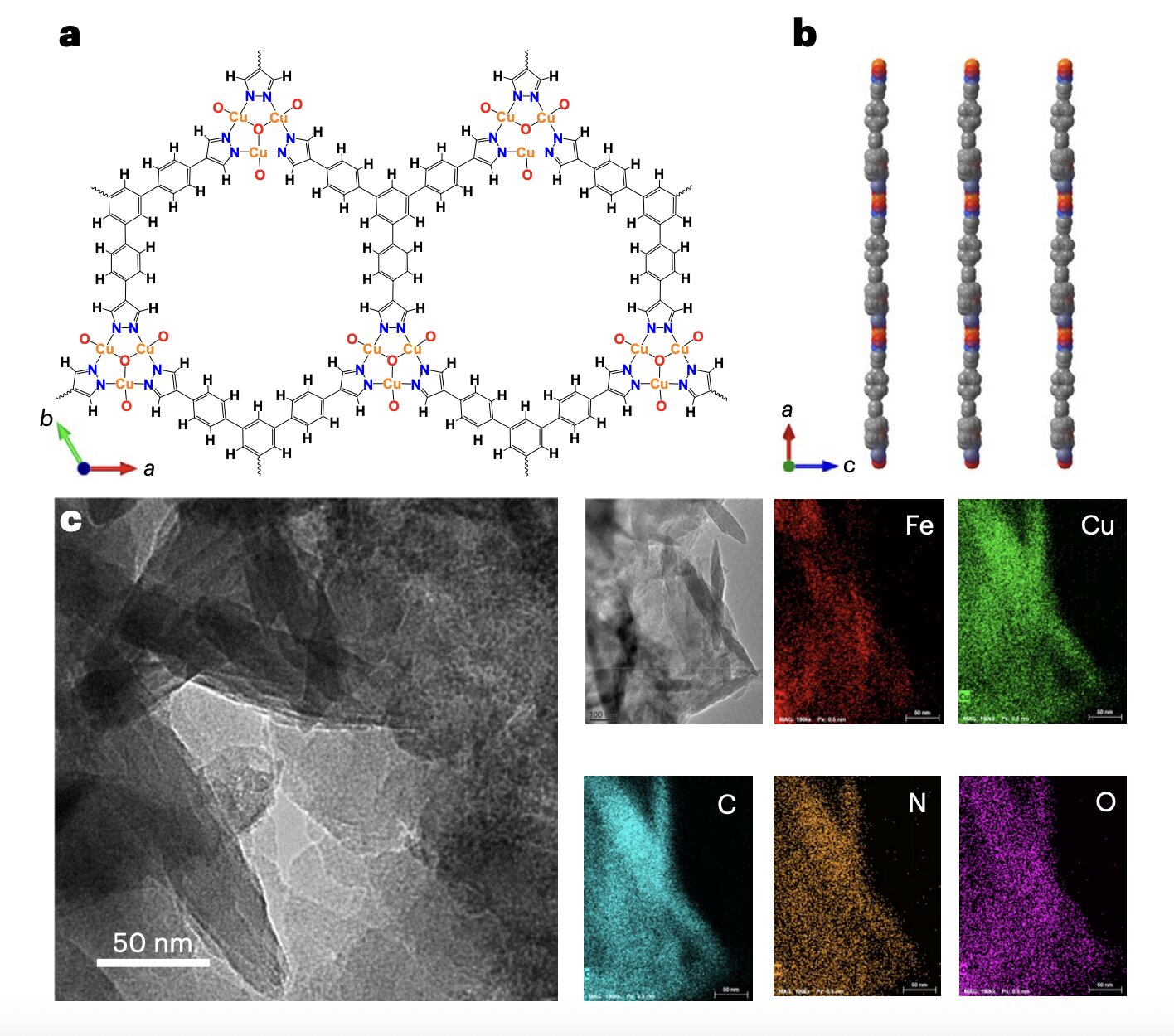
The electrosynthesis of urea hinges on one critical event: C–N coupling—the joining of a carbon atom from CO₂ with a nitrogen-containing intermediate. In this new system, the carbon comes from *adsorbed CO₂ (CO₂) and the nitrogen intermediate is NHNH, formed through semi-hydrogenation of N₂.
In the team’s proton-scarce environment, the CO₂ and NHNH species remain on the surface of a carefully chosen catalyst: an ultrathin two-dimensional metal–azolate framework, adorned with cyclic heterotrimetal clusters. This catalyst excels at stabilizing the intermediates and bringing them into close proximity, enabling the elusive bond formation that leads to urea.
“By limiting excessive hydrogenation of nitrogen,” the researchers explain, “we create ideal conditions for the precise coupling of carbon and nitrogen atoms.”
Impressive Results and Pure Product
The results are striking. When fed with pretreated flue gas—containing 85% N₂ and 15% CO₂—the electrolyzer achieved a Faradaic efficiency of 65.5%, a measure of how efficiently electricity is used to drive the desired reaction. Just as importantly, the system produced no ammonia or other liquid byproducts, yielding pure urea in aqueous solution.
Operating at a low cell voltage of 2.0 V, the electrolyzer reached a current of 100 mA, with a urea production rate of 5.07 grams per gram of catalyst per hour, or 84.4 millimoles per gram of catalyst per hour. These numbers are not just scientifically impressive—they’re industrially relevant.
Even more compelling, the team demonstrated continuous operation for at least 30 hours, producing a 6.2 wt% urea solution and recovering over 1.24 grams of pure solid urea. For a proof-of-concept setup, that level of sustained performance suggests serious scalability.
From Flue Gas to Fertilizer: Environmental and Economic Impacts
Perhaps the most exciting aspect of this innovation is its use of flue gas—a waste stream that would otherwise contribute to pollution and climate change. By upcycling this exhaust into a valuable agricultural product, the new method offers a powerful example of circular economy thinking.
“The use of pretreated flue gas as a direct feedstock significantly reduces input costs,” the researchers note, “while the high reaction rate and selectivity contribute to lower system and operational costs.”
Compared to the traditional ammonia-based route, this method consumes less energy, emits less CO₂, and potentially cuts the capital investment required for large-scale urea production plants. If further developed, it could help countries with limited natural gas or ammonia infrastructure to produce their own fertilizers domestically, boosting food security and economic resilience.
Challenges Ahead and the Road to Commercialization
While the breakthrough is undoubtedly promising, several challenges remain before this technology can be deployed at scale. Solid-state electrolyzers, while efficient and selective, often suffer from durability issues, and the cost of the advanced catalysts must be brought down through further research and materials optimization.
Scaling the process from laboratory bench to industrial reactor will also require innovations in reactor design, gas pretreatment, and process control. Moreover, integrating this new method into existing fertilizer production and distribution systems will necessitate careful planning and coordination.
Nevertheless, the implications are profound. If this approach proves viable at industrial levels, it could redefine the global fertilizer market, displacing ammonia-based urea production with a cleaner, cheaper, and more sustainable method.
The Bigger Picture: Toward Sustainable Chemistry
The story of urea’s reinvention is part of a broader trend in chemistry and materials science—a movement toward green synthesis, electrification, and carbon utilization. Around the world, researchers are racing to reengineer chemical processes to be compatible with renewable electricity, recycled carbon, and climate goals.
In this context, the Sun Yat-Sen University team’s work offers a model for how traditional chemistry can be rethought from first principles. Instead of accepting ammonia as a prerequisite, they questioned the assumption and asked: “What if we could make urea directly from the air?”
That kind of bold thinking, grounded in careful experimentation and guided by sustainability, is what the future of chemistry demands.
Conclusion: A Brighter Future for Urea
Albert Einstein once said, “We cannot solve our problems with the same thinking we used when we created them.” The traditional methods for making urea helped feed the world, but they also helped heat the planet. As the global population grows and the climate crisis deepens, the need for cleaner, smarter chemistry has never been more urgent.
Thanks to cutting-edge work like that from Sun Yat-Sen University, the path forward is becoming clearer. With clever catalysts, careful engineering, and a willingness to rethink the basics, we may soon have a way to produce urea that’s not only efficient and affordable—but also sustainable.
And that’s not just good news for the fields we fertilize. It’s good news for the entire planet.
Reference: Yan-Chen Liu et al, Electrosynthesis of pure urea from pretreated flue gas in a proton-limited environment established in a porous solid-state electrolyte electrolyser, Nature Nanotechnology(2025). DOI: 10.1038/s41565-025-01914-3