At the dawn of the universe, there was darkness, chaos, and unimaginable heat. Then, within the first few minutes of the Big Bang, something extraordinary happened: the first atoms began to form. And of all the atoms that emerged from that fiery crucible, one was born first—hydrogen. Simple, elusive, and omnipresent, hydrogen is the primordial element, the first child of the cosmos. It emerged before stars, before galaxies, before time seemed to matter.
This origin story alone would give hydrogen a special place in the pantheon of elements. But hydrogen’s uniqueness goes far beyond its age. It defies categorization, refuses to fit neatly into the boxes of modern chemistry, and exhibits behaviors so diverse and surprising that scientists have debated its placement on the periodic table for more than a century.
In this article, we dive deep into the scientific, historical, quantum, and even philosophical reasons why hydrogen is so singular in the world of chemistry. We explore its cosmic origins, atomic structure, chemical versatility, industrial importance, and role in modern energy systems. Hydrogen is not just the lightest of elements—it is, in many ways, the most profound.
Hydrogen’s Atomic Simplicity—And Its Quantum Depth
Hydrogen is the simplest atom possible: one proton, one electron, and, in its most common form, no neutrons. This simplicity might suggest predictability or even boredom. But don’t be fooled. Because of its simplicity, hydrogen is also the most sensitive to the subtleties of quantum mechanics.
In hydrogen’s one-electron system, the effects of quantum theory are not just noticeable—they are dominant. The Schrödinger equation, which governs the behavior of particles at the atomic scale, finds its most elegant and exact solution in the hydrogen atom. Physicists have studied hydrogen to test and refine the laws of quantum electrodynamics (QED), the theory that describes how light and matter interact.
Yet even within this apparent simplicity, hydrogen’s behavior is rich and varied. Its lone electron is easily stripped away, making hydrogen a reactive and energetic participant in chemical reactions. At the same time, its positively charged nucleus—the proton—can bond with electrons or participate in nuclear fusion, giving rise to new elements.
A Periodic Table Rebel
In any periodic table, hydrogen is a visual oddity. Positioned at the top of Group 1, above lithium, it is technically classified among the alkali metals. But hydrogen is no metal. It’s a gas at room temperature. It doesn’t form the same ionic compounds as its groupmates. It doesn’t behave like sodium or potassium.
At the same time, hydrogen only needs one more electron to complete its valence shell—just like the halogens in Group 17. So in some ways, it also resembles fluorine or chlorine. Yet it also doesn’t behave exactly like a halogen, either.
Some periodic tables even display hydrogen floating above the table altogether, unattached to any group, like a solitary philosophical observer refusing to conform.
Hydrogen’s placement is debated because its behavior is too multifaceted for any one category. It can donate its single electron and act like a metal, or it can accept an electron and behave like a non-metal. It can share its electron in covalent bonds, form bridges between atoms, and create exotic states like hydrides. No other element is so versatile in how it bonds and behaves.
Isotopic Oddities: Hydrogen’s Family of Twins
Another trait that sets hydrogen apart is its isotopic diversity. While every element has isotopes—versions with differing numbers of neutrons—hydrogen’s are particularly unique and useful.
- Protium (¹H): This is the most common form of hydrogen, consisting of a single proton and a single electron. It has no neutrons and makes up more than 99.98% of natural hydrogen.
- Deuterium (²H or D): This heavier isotope includes one proton and one neutron. Deuterium is stable and found in small amounts in nature. Water that contains deuterium is known as “heavy water,” and it has applications in nuclear reactors and analytical chemistry.
- Tritium (³H or T): This radioactive isotope has one proton and two neutrons. Tritium is rare and used in nuclear fusion research and as a tracer in biochemical studies. It decays with a half-life of about 12.3 years.
No other element has stable isotopes that vary so dramatically in mass. Deuterium is literally twice as heavy as protium. This difference in mass affects physical properties, reaction kinetics, and even biological processes. For instance, enzymes can distinguish between hydrogen and deuterium, slowing or altering metabolic reactions.
Hydrogen’s isotopic versatility makes it essential in tracing chemical pathways, modeling planetary formation, and developing nuclear technologies.
The Star-Maker: Hydrogen Powers the Universe
Stars shine because hydrogen fuses. Deep within the hearts of stars, immense pressure and temperature overcome the repulsion between hydrogen nuclei, allowing them to merge into helium in a process known as nuclear fusion.
This fusion releases staggering amounts of energy—the very energy that lights the sky, warms the Earth, and provides the conditions for life. In our own sun, over 600 million tons of hydrogen are fused into helium every second. This process powers solar radiation, fuels photosynthesis, and drives weather systems.
Fusion doesn’t just create light—it creates elements. All the heavier elements in the universe, from carbon and oxygen to gold and uranium, were forged in the bellies of stars that began their lives by burning hydrogen. When those stars died, they scattered their elemental guts across the cosmos, seeding new worlds, new suns, and the ingredients for life.
In this way, hydrogen is the original fuel of creation, the spark that ignites stars and the foundation of cosmic alchemy.
Hydrogen in Chemistry: The Chameleon of Bonding
In chemistry, hydrogen exhibits a startling range of behaviors. It can form ionic bonds, covalent bonds, metallic-like bonds, and even unusual interactions like hydrogen bonds. This adaptability makes hydrogen a keystone of organic and inorganic chemistry alike.
- Covalent bonding: In molecules like water (H₂O), methane (CH₄), and ammonia (NH₃), hydrogen forms covalent bonds by sharing its lone electron. These compounds are the backbone of organic chemistry and biology.
- Ionic bonding: In metal hydrides such as sodium hydride (NaH), hydrogen acts like a negatively charged ion (H⁻), bonding with a metal in a manner similar to a halide.
- Hydrogen bonding: This weak but essential interaction occurs when hydrogen, already covalently bonded to a highly electronegative atom like oxygen or nitrogen, interacts with another electronegative atom nearby. Hydrogen bonding is crucial in biology: it holds DNA strands together, stabilizes protein structures, and affects the boiling and freezing points of water.
- Hydrogen as a reducing agent: In many chemical reactions, hydrogen donates electrons and reduces other substances. It plays a central role in industrial processes like the Haber-Bosch synthesis of ammonia and the refining of petroleum.
The sheer variety of chemical behaviors exhibited by hydrogen illustrates its chameleon-like nature and explains its central role in countless reactions and processes.
Hydrogen in Water: Life’s Elixir
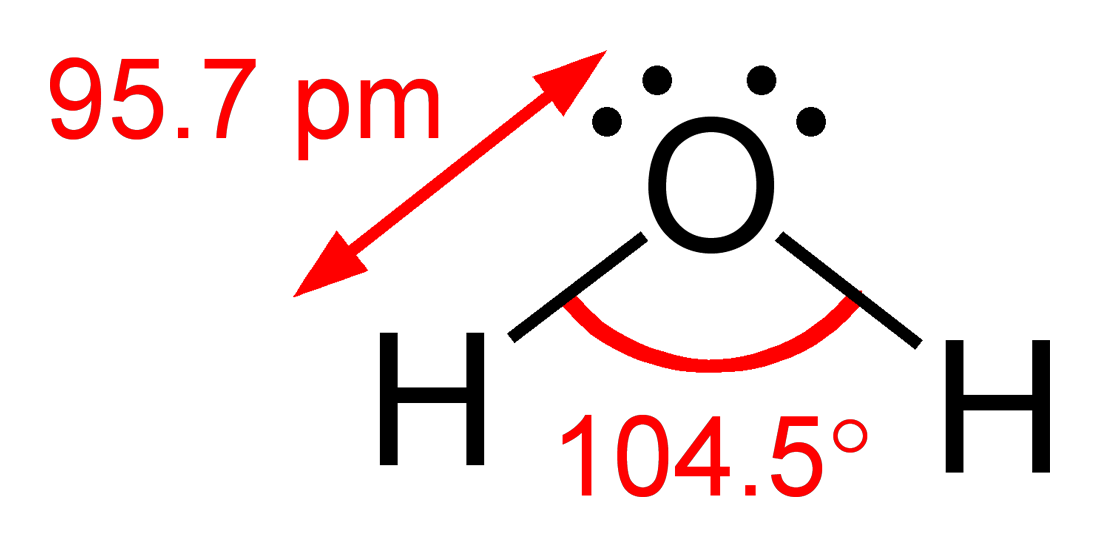
Water, the solvent of life, is a compound of hydrogen and oxygen. Yet the role of hydrogen in water is anything but passive. Its small size and ability to form hydrogen bonds give water many of its unusual and life-sustaining properties.
Water has an unusually high boiling point and specific heat for such a small molecule. It exists in all three states—solid, liquid, and gas—within the range of temperatures experienced on Earth. These characteristics stem from the hydrogen bonds between water molecules, which require additional energy to break.
When water freezes, the hydrogen bonds cause its molecules to arrange into a hexagonal lattice, making ice less dense than liquid water. That’s why ice floats—a rare and life-critical phenomenon that insulates aquatic life in cold climates.
Hydrogen’s interactions with oxygen in water also enable auto-ionization, giving rise to hydronium (H₃O⁺) and hydroxide (OH⁻) ions. This underpins the concept of pH and acid-base chemistry, governing everything from cellular respiration to ocean health.
Without hydrogen, water would not exist. Without water, neither would life.
The Hydrogen Economy: Energy of the Future?
As the world looks to transition from fossil fuels to sustainable energy, hydrogen has emerged as a potential game-changer. It’s abundant, clean-burning, and highly energetic. When hydrogen reacts with oxygen, it produces only water and energy—no carbon dioxide, no pollution.
Hydrogen fuel cells convert hydrogen’s chemical energy directly into electricity, powering everything from cars to spacecraft. Unlike batteries, fuel cells can be refueled quickly and provide long-lasting energy with no emissions.
Hydrogen is also being explored as a way to store renewable energy. When solar or wind power produces excess electricity, that energy can be used to electrolyze water, splitting it into hydrogen and oxygen. The hydrogen can then be stored and later used as fuel.
But the hydrogen economy faces challenges. Producing pure hydrogen often requires energy from fossil fuels, unless it’s made via electrolysis using renewables. Storing and transporting hydrogen safely also poses engineering hurdles, as it’s a highly flammable gas and has the smallest molecular size of any element—making it prone to leakage.
Despite these obstacles, hydrogen remains a tantalizing prospect: the clean fuel that could power a decarbonized world, bridging the gap between today’s energy needs and tomorrow’s sustainable solutions.
Philosophical and Symbolic Importance
Hydrogen is not just scientifically unique—it has philosophical weight. As the first element in the periodic table, hydrogen represents beginnings. It symbolizes simplicity, purity, and potential. It’s the source from which complexity grows, the raw material of stars and life.
Hydrogen’s presence in both the farthest galaxies and the deepest parts of our bodies connects the human experience to the cosmos. The same element that fuels the sun flows in our blood, dances in our neurons, and whispers through our breath.
In spiritual and metaphysical thought, hydrogen has been equated with the fundamental essence of being. It is the alpha of matter, the start of all chemical possibility, the simplest yet most profound atom in existence.
As we reach further into the depths of space and the intricacies of biology, hydrogen continues to be our guide—a tiny particle with a massive legacy.
Conclusion: The Smallest Giant
Hydrogen is, in many ways, the ultimate paradox. It is the lightest, simplest, and most abundant element, yet its behavior is richer and more complex than almost any other. It refuses to be boxed in by periodic categories. It wears many chemical hats. It powers stars and cars. It links quantum mechanics with cosmology, and fuels the reactions that make life possible.
To study hydrogen is to peer into the fundamental fabric of reality. To understand it is to begin to understand everything—from the birth of the universe to the future of energy on Earth. In hydrogen’s ghostly glow, we see the past and the future, the macro and the micro, the material and the miraculous.
Hydrogen is more than just element number one. It is chemistry’s original rebel, the seed of stars, the breath of life, and the hope of a cleaner world. In its small frame, it carries the weight of the universe.