For over a century, one fundamental principle has reigned in the world of organometallic chemistry: the 18-electron rule. This chemical rule of thumb states that transition metal complexes are most stable when they are surrounded by 18 formal valence electrons—a concept that has shaped research in catalysis, materials science, and beyond. But what happens when the rule is broken?
A groundbreaking study from the Okinawa Institute of Science and Technology (OIST), in collaboration with scientists from Germany, Russia, and Japan, has done just that—challenging the long-held belief that 18 electrons are the limit. The team has successfully synthesized a stable 20-electron ferrocene derivative, a compound that opens the door to new possibilities in chemistry and material science.
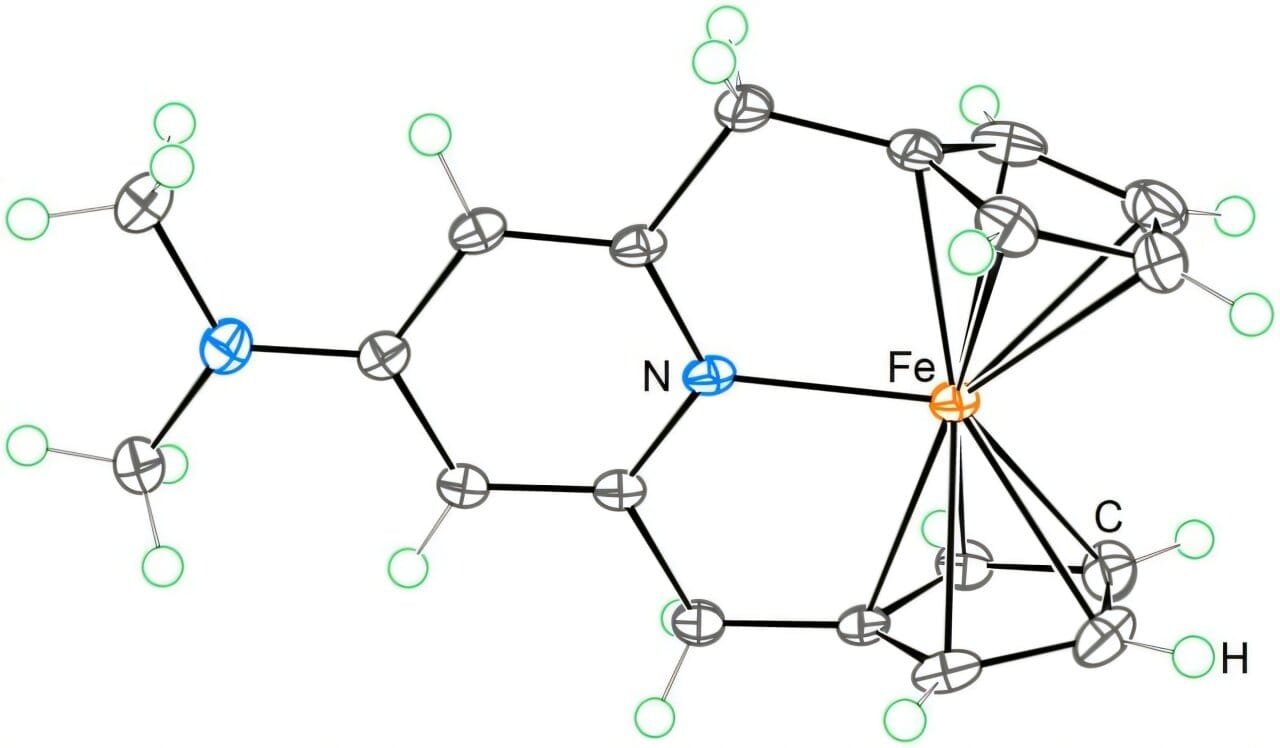
“For many transition metal complexes, they are most stable when surrounded by 18 formal valence electrons. This has been a cornerstone of chemical theory,” said Dr. Satoshi Takebayashi, lead author of the study published in Nature Communications. “Now, we have shown for the first time that it is possible to synthesize a stable 20-electron ferrocene derivative.”
This achievement doesn’t just push the boundaries of what we know about chemical stability—it rewrites the very rulebook on organometallic compounds.
Ferrocene: The Classic Compound with a New Twist
To understand the significance of this new discovery, one must first appreciate the role of ferrocene in the history of chemistry. First synthesized in 1951, ferrocene was an unexpected marvel, its stability and “sandwich” structure—where an iron atom is flanked by two organic cyclopentadienyl rings—revolutionizing our understanding of metal-organic bonding.
Ferrocene’s discovery catapulted a new field of study: organometallic chemistry. It not only helped explain how metals and organic compounds could interact, but it also led to the 1973 Nobel Prize in Chemistry for its inventors. Since then, ferrocene and its derivatives have become a cornerstone of research in fields ranging from catalysis to materials science.
But despite its many applications, ferrocene’s utility has been constrained by the 18-electron rule. The structure, while stable, has traditionally been limited in its ability to accept or donate electrons in chemical reactions.
Now, thanks to the work of Dr. Takebayashi’s team, that limitation has been shattered.
Expanding the Boundaries: The Power of 20 Electrons
In this new study, the team introduced a novel ligand system to stabilize a ferrocene derivative with 20 valence electrons—a feat that was previously deemed improbable. These additional electrons not only break the traditional mold of organometallic chemistry but also unlock a suite of exciting properties that could propel new scientific innovations.
“More importantly, the additional two valence electrons induced an unconventional redox property that holds potential for future applications,” Dr. Takebayashi explained.
Redox reactions, which involve the transfer of electrons between molecules, are essential to a wide range of chemical processes. Ferrocene has already been used in such reactions, but its ability to undergo oxidation and reduction has traditionally been limited to a narrow range. The team’s new compound, however, opens up new oxidation states, enabling a broader spectrum of electron transfer.
This new redox behavior could make the 20-electron ferrocene derivative a game-changer in catalysis—enhancing its role in chemical reactions, particularly in energy storage, sustainable chemistry, and chemical manufacturing.
Rebuilding the Rules of Stability
The implications of this discovery stretch beyond ferrocene itself. By understanding how to manipulate and stabilize compounds with more than 18 electrons, chemists can design molecules with tailor-made properties—ones that might not have been possible within the confines of conventional chemistry.
“This breakthrough gives us a new platform for future innovation,” said Dr. Takebayashi. “It expands the conceptual toolkit available to chemists, enabling us to create novel compounds with entirely new characteristics.”
For example, the newly synthesized 20-electron ferrocene derivative could serve as the basis for green catalysts that are more efficient and environmentally friendly, as well as next-generation materials for technologies like solar cells, pharmaceuticals, and advanced medical devices.
Ferrocene’s Legacy: From the Lab to the Real World
Ferrocene derivatives have already made significant contributions to numerous technologies, such as solar cells and medical devices, and have played a key role in the development of modern catalysis. By expanding the versatility of ferrocene through the introduction of the 20-electron derivative, the team at OIST is opening up a whole new set of potential applications that could drive scientific progress in countless fields.
The discovery could also pave the way for more sustainable chemistry. As energy storage becomes increasingly critical in our fight against climate change, the ability to design better, more efficient catalysts and materials will be essential. By allowing ferrocene to access new redox states, the compound could play a pivotal role in the development of next-generation energy solutions.
The Organometallic Chemistry Group at OIST, which led this research, has always been interested in uncovering the fundamental principles that govern metal-organic interactions. This breakthrough, which challenges conventional chemical rules, is just one step in their ongoing quest to unlock unconventional compounds that defy traditional expectations.
The Future of Chemical Innovation
By breaking the 18-electron rule, OIST’s team has expanded the possibilities of what can be achieved with organometallic chemistry. Their discovery demonstrates how a better understanding of chemical stability can unlock new avenues for molecular design, leading to materials and catalysts with enhanced properties.
As researchers continue to experiment with novel metal-organic compounds, the potential for new discoveries is limitless. This breakthrough could be just the beginning of a new era in which scientists push the boundaries of chemistry, creating substances with unique characteristics that can transform industries, from energy to medicine.
With this innovation, chemists can begin to design molecules not by the rules, but by the possibilities—a new frontier in science that holds exciting promise for the future.
Reference: Satoshi Takebayashi et al, From 18- to 20-electron ferrocene derivatives via ligand coordination, Nature Communications (2025). DOI: 10.1038/s41467-025-61343-7