In the realm of molecular architecture, few puzzles are more fascinating—and frustrating—than that of enantiomers. These elusive pairs of molecules are chemically identical, but mirror images of each other—like your left and right hand. And just as you can’t comfortably wear a left shoe on your right foot, your body can’t always “wear” both enantiomers the same way. In fact, one enantiomer can heal, while the other might harm.
This seemingly subtle distinction has profound implications in medicine. More than half of all FDA-approved drugs are chiral, meaning they come in enantiomeric forms. And within those, often only one enantiomer is therapeutically effective, while the other is inert—or worse, toxic. The stakes are high. In some notorious historical cases, like the tragedy of thalidomide in the 1950s and 60s, the wrong enantiomer caused severe birth defects while the intended one alleviated nausea in pregnant women.
So how do you separate two molecules that are chemically identical in every measurable way—except that they are not superimposable?
That’s exactly the challenge tackled by a team of researchers at the University of Illinois Urbana-Champaign. In a newly published paper in the Journal of the American Chemical Society, they unveil a game-changing advance in enantiomer separation—one that’s not only more efficient, but also more sustainable. At the heart of this breakthrough lies a family of electrically controllable chiral polymers derived from ferrocene—a sandwich-shaped molecule known for its unique electronic properties.
And the key to their innovation? A kind of molecular handshake that works only in one direction.
Mirror Molecules and a Molecular Dilemma
To the naked eye—and even to most analytical tools—enantiomers are indistinguishable. Their masses, boiling points, and other basic properties are the same. What sets them apart is chirality, a geometric property that renders two molecules non-superimposable, even though all their atoms are in the same relative positions.
This makes them particularly tricky to separate. The traditional method involves introducing a “chiral environment”—a system or surface that itself has handedness—capable of distinguishing one enantiomer from the other by preferentially binding or reacting with it.
But current industrial-scale chiral separations are not ideal. They’re time-consuming, require massive volumes of solvents, and generate equally massive amounts of chemical waste. “Pharmaceutical separations can often be very costly and chemically wasteful,” says Dr. Xiao Su, the study’s senior author and a chemical and biomolecular engineering professor at Illinois.
So Su’s team set out to design a smarter, cleaner way to tackle the enantiomeric bottleneck—one molecule at a time.
Electric Elegance: Harnessing Chirality Through Ferrocene
Ferrocene is an organometallic marvel—composed of two cyclopentadienyl rings sandwiching an iron atom. It’s stable, highly customizable, and most importantly, redox-active—it can donate or accept electrons in a controlled way. This makes it a perfect candidate for designing molecules that respond to electric fields, a feature rarely exploited in chiral separations.
“We wanted to achieve a more sustainable but still effective enantioselective separation by developing a chiral interface that can selectively capture one enantiomer over the other—and be turned on and off by electricity,” explains Jemin Jeon, the study’s co-first author and former Ph.D. student in Su’s lab.
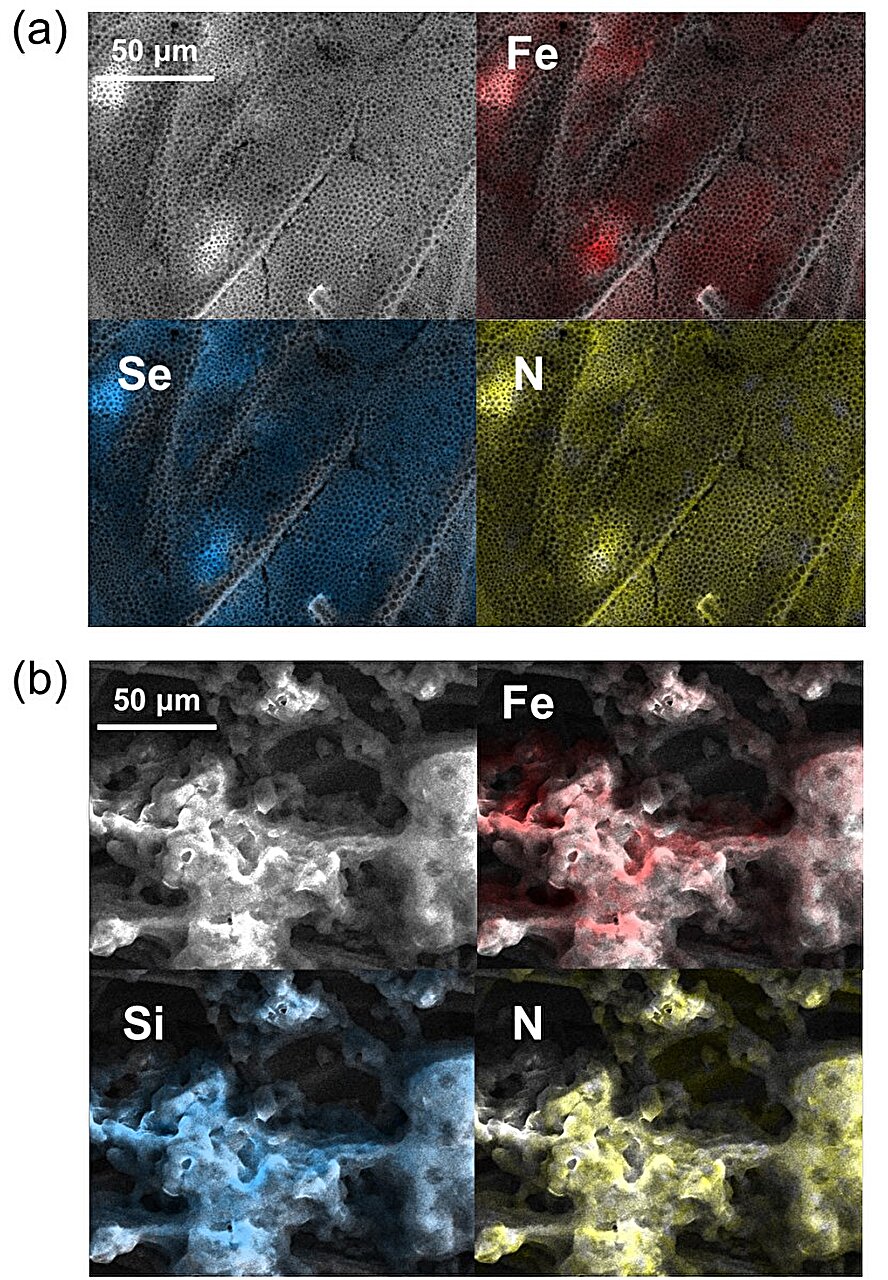
To do that, the team modified the ferrocene’s molecular framework by adding methyl and selenium phenyl groups, which introduced planar chirality. Unlike traditional point chirality—where the “handedness” is centered on a single atom—planar chirality arranges the chiral elements across a two-dimensional surface, allowing for a broader and more nuanced interaction with target enantiomers.
This design forms the crux of what the researchers call a “chiral interface”: a surface that not only distinguishes between enantiomers but also binds to them selectively based on their handedness.
But it gets even more elegant: this interface is electrochemically responsive. That means a voltage can switch the polymer’s enantiomer-binding properties on or off—like flicking a light switch to selectively capture or release mirror molecules.
From Lab Bench to Breakthrough: Proof of Concept
The team tested their new polymers using racemic mixtures—50/50 blends of both enantiomers—of amino acids, which are among the most common chiral molecules in pharmaceuticals. When they applied an electric current to the system, they found that the polymers could selectively attract and hold one enantiomer over the other. With careful tuning, the system reached enantiomer purities of up to 99%.
What’s especially promising is the reversibility and reusability of this system. Once the desired enantiomer is captured, an electric signal can release it. No need for harsh chemicals or additional solvents. “The uniqueness of these polymers is that they are not only chiral, but they’re chiral and electrochemically responsive,” says Su. “It’s a completely new application for electrochemical separations.”
This advance marks a dramatic improvement over point-chiral systems previously used by the same group. Planar chiral ferrocene polymers proved to have significantly better enantioselectivity, opening the door for more precise and scalable separations.
A Sustainable Vision for the Future of Drug Development
Beyond the technical achievement lies a larger vision: transforming how the pharmaceutical industry thinks about purity, waste, and sustainability. Current drug manufacturing practices for chiral compounds often rely on high-performance liquid chromatography (HPLC) with expensive and wasteful chiral columns. These columns need to be replaced frequently and use large quantities of organic solvents—many of which are toxic or non-recyclable.
But the electrochemical method championed by Su’s team offers a radically cleaner alternative. By using electricity—a renewable and easily controlled input—rather than chemical gradients, the process slashes solvent use and reduces waste generation. And because the polymers are reusable and robust, they minimize material costs over time.
“The separation of these enantiomers requires large systems that use a lot of solvent and which generate a lot of chemical waste,” Su explains. “By doing this process electrochemically, you can reduce the waste and the chemicals that are being used.”
That’s a boon not only for pharmaceutical companies trying to reduce their environmental footprint, but also for researchers in early-stage drug discovery who often struggle with the costs and complexity of chiral analysis.
The Molecular Frontier: What Comes Next?
While the team’s current work focused on amino acids, the implications go far beyond. Many drugs—from antidepressants to cancer therapeutics—exist in chiral forms, and new molecular scaffolds are being developed every day in labs around the world.
“This is just a beginning for the design of redox-active chiral interfaces and electrochemical systems,” says Jeon. “We believe there are unlimited opportunities in pursuing these concepts for the enantioselective separations of a wider group of molecules, including valuable pharmaceuticals.”
Indeed, the ability to tailor the ferrocene-based polymers to specific target molecules means that this approach could be customized for almost any chiral drug. In the future, we could see compact, plug-and-play devices for chiral separation used in hospitals, labs, or even remote field settings—bringing precision drug screening closer to the point of care.
And with continued refinement, these materials might not just separate enantiomers—they might also help identify them. Combining molecular recognition with electrochemical readouts could lead to powerful diagnostic tools that sense chiral imbalances in real time, a potential boon for monitoring disease progression, metabolic disorders, or even the gut microbiome.
Chemistry with a Conscience
This work exemplifies a broader movement in chemical engineering: the shift toward greener, smarter technologies that don’t compromise performance. It’s not enough anymore to just get the job done; the challenge is to do it with elegance, efficiency, and environmental responsibility.
The University of Illinois team has shown that it’s possible to navigate one of chemistry’s most difficult terrains—the separation of mirror-image molecules—using tools that are both high-tech and low-waste. And they did it by reimagining a humble molecule, ferrocene, as a dynamic scaffold for molecular discrimination.
In doing so, they’ve electrified the field—literally—and set a new standard for what chiral separations can look like in the 21st century.
So the next time you take a painkiller or a prescription medication, spare a thought for the tiny molecular dance happening inside—and the researchers who are making sure that the right hand, not the left, takes the lead.
Reference: Jemin Jeon et al, Planar Chiral Metallopolymers for Electrochemically Mediated Enantioselective Separations, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c01571