Step into your home, office, or favorite café, and you’re surrounded by more than walls, windows, and furnishings. You’re swimming in an invisible sea of chemical compounds—some benign, others potentially harmful, many still a mystery. From polished floorboards to scented candles, from your skin to your sofa, almost everything in a modern indoor environment either emits or reacts with volatile compounds. We now know that the very act of being indoors triggers a complex chemical ballet.
Humans spend around 90% of their time indoors. This staggering statistic raises a vital question: what exactly are we breathing? Far from being an inert or sterile space, the air inside a room is a chemically dynamic environment, and we—as moving, metabolizing organisms—are key players in shaping that environment. Recent research has shed light on a surprising and underexplored phenomenon: how the personal care products we apply—lotions, perfumes, deodorants—can alter the fundamental chemistry of the air surrounding us.
And not just in a vague or general way. We now know that every person carries a sort of invisible “oxidation field,” a reactive zone influenced by our skin chemistry and the ambient air. And this self-generated cloud changes significantly with just a spritz of perfume or a layer of lotion.
Ozone, Indoors? And Why It Matters
It may sound counterintuitive, but ozone—best known as the protective layer in the upper atmosphere—is also present indoors, introduced mainly through ventilation or infiltration from the outdoors. While outdoor ozone can play a protective role against harmful solar radiation, indoor ozone becomes a major player in reactive indoor chemistry.
Once inside, ozone doesn’t just linger idly. It reacts with other molecules, many of which are emitted by our own skin or clothing. These reactions can give rise to secondary pollutants—compounds that weren’t in the room to begin with but emerge from interactions between ozone and other chemicals.
Among the most important of these reactions are those involving the unsaturated lipids on human skin—especially squalene, a waxy substance that protects and lubricates our skin. When ozone reacts with squalene, it can generate a host of volatile byproducts, including hydroxyl (OH) radicals—highly reactive molecules that drive much of the oxidative chemistry in both outdoor and indoor environments.
But what happens when this chemistry is disrupted—not by industrial emissions or open flames, but by the lotions and fragrances we apply to our own skin?
The Human Oxidation Field: An Air Chemistry Ecosystem
The concept of a “human oxidation field” might sound like science fiction, but it’s very real. First coined by atmospheric chemists to describe the reactive chemical halo that surrounds each person, this field is driven largely by the reaction of skin-emitted compounds with ozone. In this space, OH radicals are created, initiating complex chains of chemical reactions.
Why does this matter? Because the oxidation field influences the chemical composition of the air we breathe, especially in the so-called breathing zone—the area just inches from our nose and mouth. Changes in this field can affect what chemicals are formed in this intimate space and how they interact with our respiratory system.
This is where the latest research, led by Jonathan Williams from the Max Planck Institute for Chemistry, becomes transformative. His team’s work, published in Science Advances, reveals how common personal care products can weaken or suppress this oxidation field, altering the types and amounts of reactive compounds in our immediate surroundings.
Body Lotion and Perfume: Chemistry That Lingers
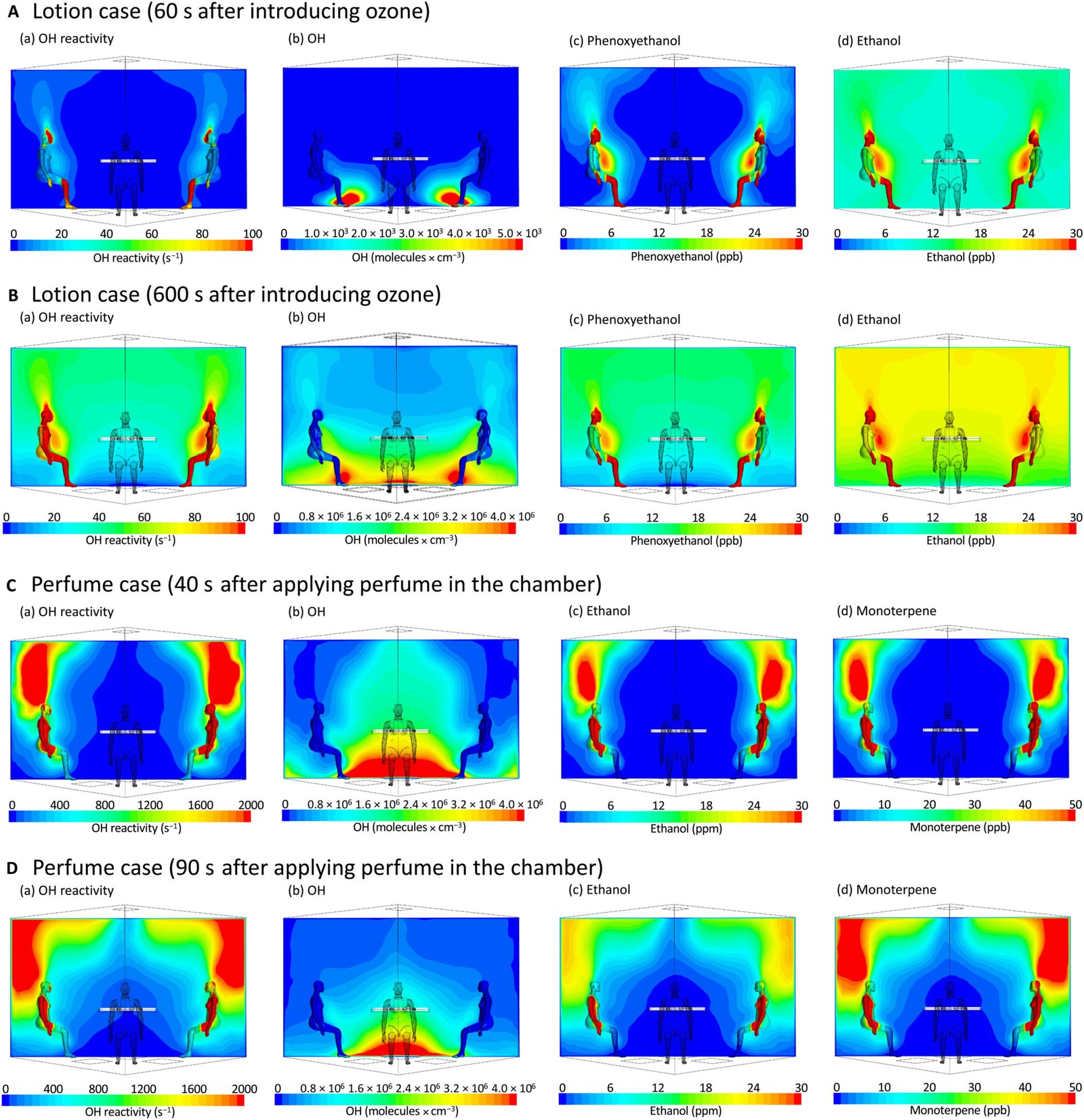
In their experiments, Williams and his collaborators tested how common personal care items like body lotion and perfume influence the oxidation field. Using a combination of controlled chamber studies and sophisticated modeling, they discovered that both lotions and fragrances significantly suppressed the formation of OH radicals near the skin.
Take perfume, for instance. Its main component—ethanol—readily reacts with OH radicals. But unlike ozone reacting with squalene, this reaction doesn’t produce more OH radicals; it uses them up. The result? A net decrease in OH concentration in the personal breathing zone.
Lotions had an even more complex effect. One explanation is similar to perfume: ingredients like phenoxyethanol react with OH but don’t regenerate it. But lotions also create a physical barrier. When applied to the skin, they dilute or block access to squalene and other reactive oils. This means ozone has fewer reactive targets, and as a result, fewer OH radicals are produced.
Fascinatingly, the research showed that the effects of perfume were shorter-lived and more localized, whereas the impact of lotion was longer-lasting and more spatially diffuse. “The application of a fragrance and a lotion together showed that fragrances impact the OH reactivity and concentration over shorter time periods, whereas lotions show more persistent effects,” explained Nora Zannoni, first author of the study.
Simulating Human Chemistry in 3D
To understand the physics and chemistry of these changes, the team took a multidisciplinary approach. Researchers from the University of California, Irvine, led by Manabu Shiraiwa, developed a cutting-edge chemical model to simulate ozone’s interaction with skin and clothing. Meanwhile, Donghyun Rim from Pennsylvania State University employed 3D computational fluid dynamics (CFD) to visualize how this chemistry evolves in space and time around a person.
The combination of models showed how the oxidation field shifts in response to various chemical inputs. For example, lotion can dampen the oxidative halo by both absorbing ozone and altering the surface chemistry of the skin. These changes are not trivial; they could influence the makeup of the air we breathe over hours, or even longer.
The Experiments: Climate Chambers and Chemical Clouds
The real-world component of the study was conducted in 2021 at the Technical University of Denmark (DTU). Four volunteers spent time in a specially designed, climate-controlled chamber where ozone was added to mimic the higher end of indoor concentrations—levels not harmful to humans but sufficient to trigger reactive chemistry.
Inside this chamber, scientists carefully measured air composition using high-precision instruments and indirect markers of OH concentration. By comparing air samples before and after the application of personal care products, they calculated how these items influenced the human oxidation field.
The results were crystal clear: both perfume and body lotion suppressed the production of OH radicals, changing the chemical makeup of the air in the breathing zone.
Implications: What Does This Mean for You?
The implications of this research stretch far beyond vanity or hygiene. We tend to think of our living spaces as controlled environments, but the chemistry occurring inches from our face is anything but stable or predictable.
For one, this study raises critical questions about the long-term effects of chronic exposure to indoor air chemistry altered by personal products. What happens when the oxidation field is routinely suppressed? Could this make us more vulnerable to certain airborne pollutants that would otherwise be broken down? Could it affect how our bodies respond to allergens or pathogens?
Moreover, as Williams notes, “If we buy a sofa from a major furniture company, it is tested for harmful emissions before being put on sale. However, when we sit on the sofa, we naturally transform some of these emissions because of the oxidation field we generate.” With the field dampened, fewer of those emissions may be chemically transformed—but what does that mean for toxicity or long-term exposure?
Another layer of complexity comes from the sheer variety of personal care products on the market. Fragrances, lotions, deodorants, and cosmetics come in thousands of formulations. Each has a different impact on the oxidation field. Yet current air quality standards and safety guidelines do not account for these nuanced interactions.
The Bigger Picture: The ICHEAR and MOCCIE Collaborations
This research is part of the ICHEAR (Indoor Chemical Human Emissions and Reactivity) Project, a multinational effort to understand how human presence influences indoor air chemistry. It’s also supported by the MOCCIE (Modeling Consortium for Chemistry of Indoor Environments) initiative based at UC Irvine and Penn State, which aims to develop accurate, predictive models of indoor chemical dynamics.
Together, these initiatives are building a new field of inquiry at the intersection of environmental chemistry, public health, and personal behavior. Their message is clear: in the chemical universe of our homes and workplaces, humans are not passive occupants—we are active chemical reactors.
A Future of Personalized Air Chemistry?
Could the future bring air-quality recommendations tailored not just to your environment but to your skincare regimen? Could architects and HVAC designers one day factor in human oxidation fields when designing indoor spaces? It may sound far-fetched, but research like this is laying the groundwork.
More immediately, these findings may inform better choices for people with respiratory conditions or chemical sensitivities. They may also guide future product formulations—perhaps developing lotions and fragrances that maintain their appeal while minimizing unwanted chemical suppression.
As science continues to uncover the hidden dimensions of indoor air chemistry, one thing is becoming clear: the next frontier of environmental health might begin not with factory smokestacks or traffic fumes, but with the bottle of lotion on your bathroom shelf.
Reference: Nora Zannoni et al, Personal care products disrupt the human oxidation field, Science Advances (2025). DOI: 10.1126/sciadv.ads7908