Anyone who’s ever flinched at an ice cube or winced through a dental drill knows the strange sensitivity of teeth. They may seem like hard, inanimate blocks of enamel, but teeth are alive—wired with nerves, pulsing with sensation, and finely tuned to the world inside our mouths. Yet according to new research from the University of Chicago, those delicate sensations we associate with modern teeth may have started not in the jaws of ancient fish—but on the outside of their bodies.
Yes, teeth, it turns out, have an origin story that is less about chewing and more about sensing the world through a prehistoric suit of armor.
A Hidden Sensory System in Prehistoric Armor
New findings published in Nature by a team led by postdoctoral researcher Dr. Yara Haridy and senior author Dr. Neil Shubin reveal that dentin—the soft tissue core of teeth—likely evolved not as a tool for feeding, but as a biological sensor embedded in the exoskeletons of ancient, armored fishes.
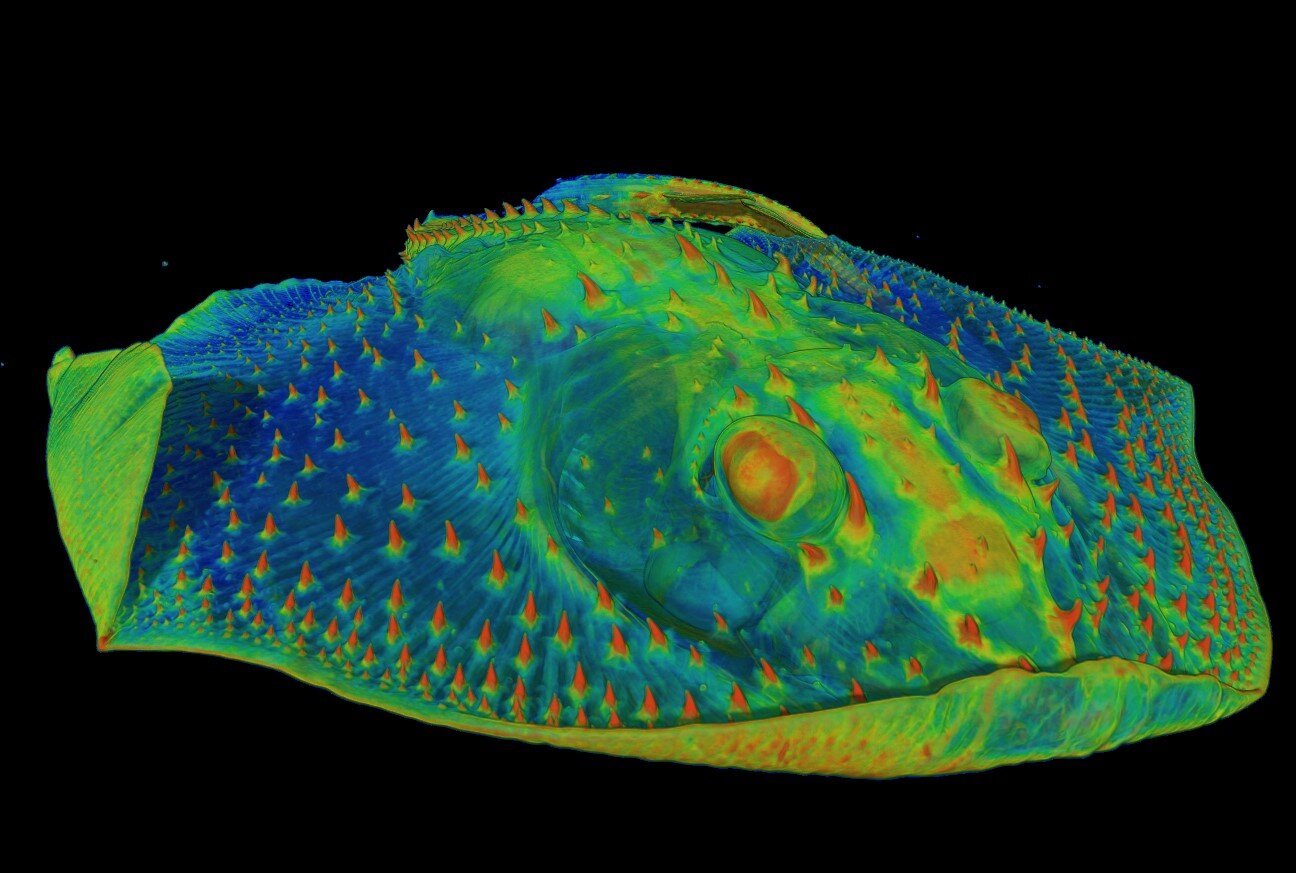
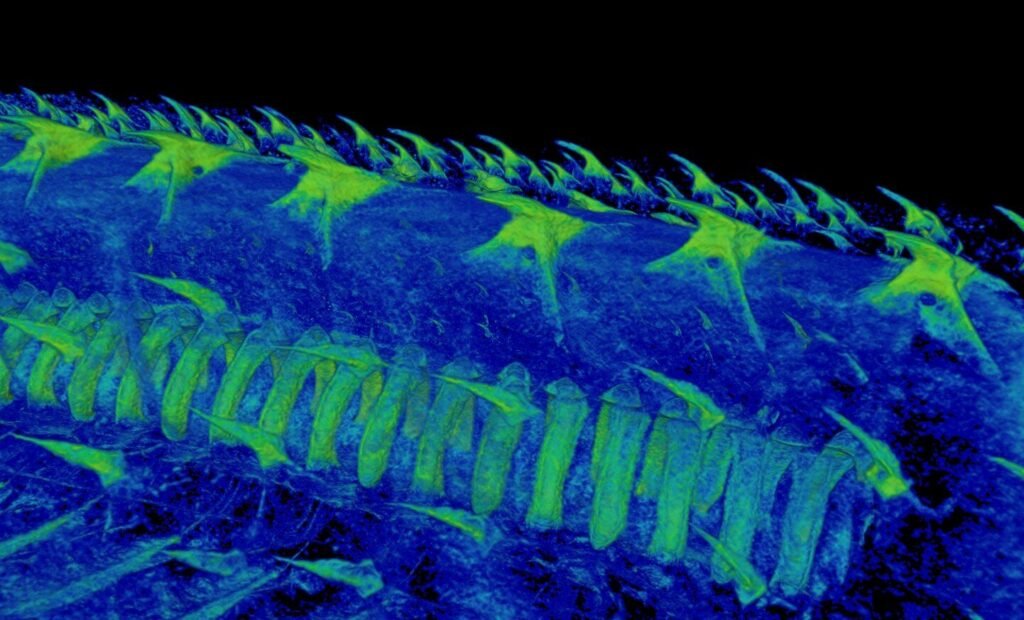
Long before vertebrates chewed, gnawed, or ground their food, their ancestors swam through predatory Paleozoic waters encased in thick plates of protective armor. These exoskeletal plates were decorated with small, bump-like structures known as odontodes—once believed to be early prototypes of teeth. Until now, the precise role of these structures was speculative. But thanks to high-resolution CT scanning and chemical analysis of fossils dating back nearly 500 million years, we now know these bumps weren’t just decoration. They were alive, and they could feel.
Inside these ancient structures lay dentin, the same tissue that makes our teeth throb when we bite into ice cream. In these armored fishes, however, the dentin wasn’t in the mouth. It was out on the body surface, likely connected to nerves. These creatures weren’t chewing—they were feeling the flow of water, detecting movement, perhaps even “seeing” with their armor in a predatory ocean world that demanded heightened awareness.
A Paleontological Surprise Party
Dr. Haridy, who led the study in Dr. Shubin’s lab, didn’t begin her investigation in search of the origins of teeth. She set out to answer a very different question: What is the earliest vertebrate in the fossil record?
That question has obsessed paleontologists for decades. The Cambrian period, which began roughly 540 million years ago, was a biological explosion that gave rise to a bizarre menagerie of creatures. Some, like the iconic Anomalocaris, were evolutionary dead ends. Others were the early architects of the vertebrate lineage that would eventually lead to fish, reptiles, birds, and mammals—including us.
Haridy’s idea was simple in theory but monumental in execution: scan Cambrian fossil specimens using high-energy synchrotron CT imaging at Argonne National Laboratory’s Advanced Photon Source, in hopes of identifying the biological hallmarks of vertebrates. Chief among those indicators? Dentin.
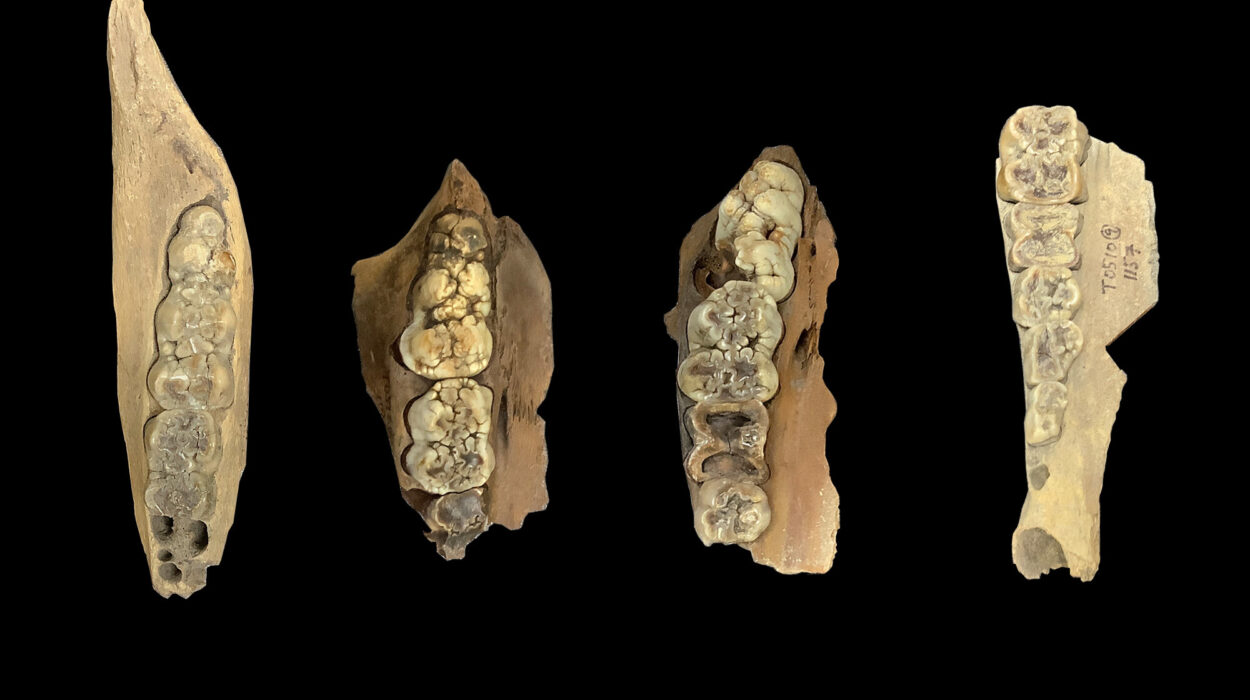
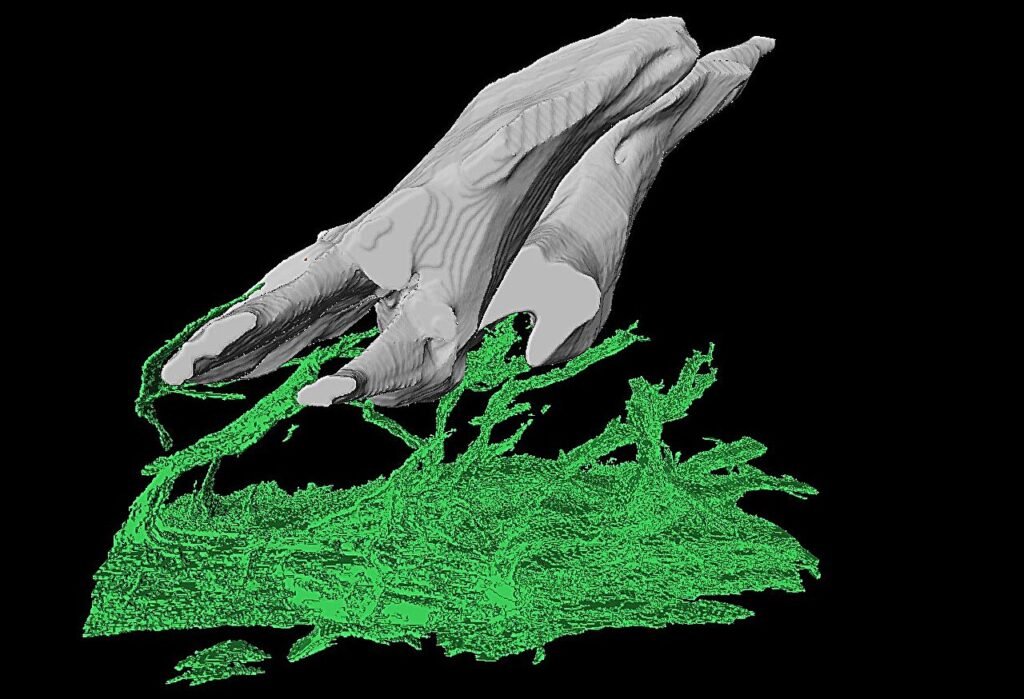
Across a marathon, all-night session at the particle accelerator, Haridy and her colleagues scanned hundreds of fossil fragments, many no larger than a sesame seed. Among them was a specimen from a genus called Anatolepis, a fossil first described in 1996 as a possible early vertebrate. To their astonishment, one sample showed features that looked strikingly like dentin-filled pores beneath the outer surface—potentially placing a vertebrate organism millions of years earlier in the fossil record than previously believed.
“There were high-fives all around,” Haridy recalled. “We thought, this might be the first tooth-like structure in vertebrate tissue from the Cambrian.”
Not a Tooth After All: A Sensory Illusion
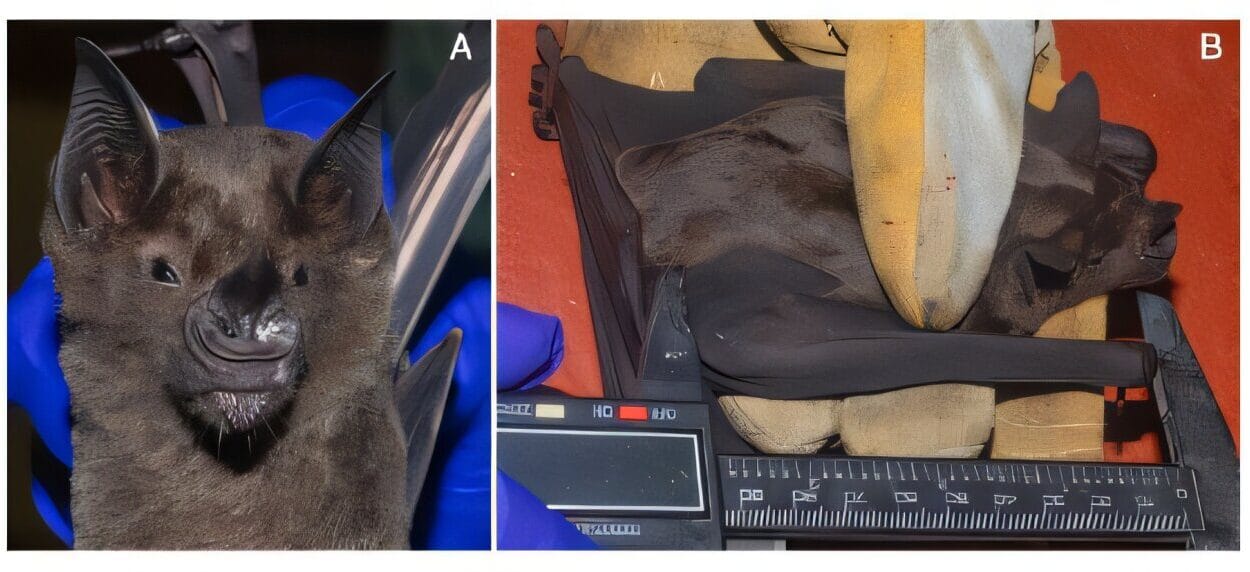
But science is slow, methodical, and skeptical—even when you’re excited. Haridy and the team needed to compare the fossil to others to ensure what they saw was truly dentin. So they assembled a massive comparative library of specimens, including fossil arthropods, modern-day crustaceans, and fish like sharks, skates, and miniature catfish—some of which Haridy raised herself.
One by one, they cross-checked structure, chemistry, and internal architecture. And that’s when things took an unexpected turn.
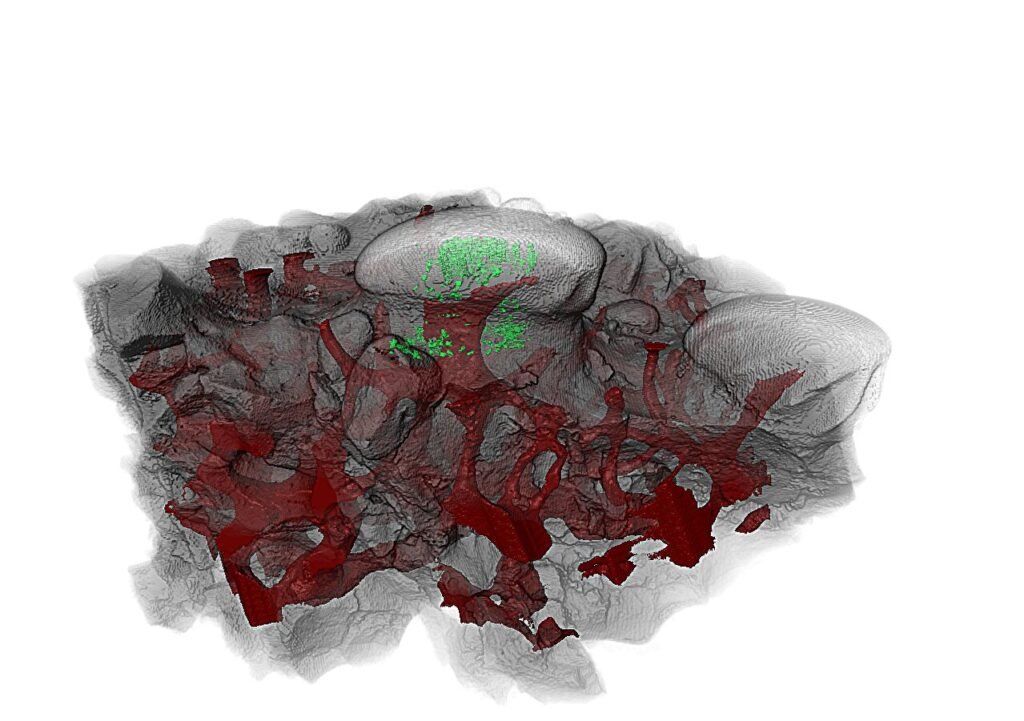
The tubules inside Anatolepis didn’t behave like vertebrate dentin when scrutinized at the microscopic level. Instead, they mirrored the sensory organs found on the shells of modern arthropods—structures called sensilla, which allow crabs, shrimp, and beetles to feel pressure, vibrations, and chemical cues.
It wasn’t just a case of mistaken identity. It was a profound evolutionary echo. What looked like tooth precursors in early vertebrates turned out to be highly evolved sensory structures in invertebrates.
Anatolepis, once hailed as a vertebrate, was actually an ancient arthropod.
Sensitive Armor in a Dangerous World
The research didn’t stop there. When Haridy turned to an Ordovician-period fossil called Eriptychius—a creature believed to be an early vertebrate from around 465 million years ago—she found something different. Its armor contained similar sensory structures to Anatolepis, but these did contain dentin. This suggested that in vertebrates, unlike in arthropods, dentin had indeed evolved inside the armored plates—not as teeth, but as sensors.
These sensitive armor bumps weren’t just evolutionary leftovers—they were crucial adaptations. In a time when the oceans teemed with predators and visibility was limited, the ability to detect changes in water flow or vibrations could mean the difference between survival and being eaten.
“We usually think of armor as something passive,” Haridy explained. “But this was interactive armor—armor that could feel. That sensory capacity might have allowed these animals to respond to their environment in real-time.”
Dr. Shubin put it more bluntly: “If you’re swimming around in a prehistoric ocean filled with predators, being able to feel the water around you could save your life.”
When Skin Became Teeth
What emerged from this project was a sweeping narrative of convergent evolution—where different lineages arrive at similar solutions through different evolutionary paths. Arthropods evolved sensilla. Vertebrates evolved dentin-lined odontodes. Both were embedded in protective armor. Both functioned as sensory organs. And both predated the evolution of jaws or teeth used for eating.
This raises a tantalizing question: If dentin first evolved as a sensory tool, how did it make its way into our mouths?
That’s where two competing evolutionary theories come into play. The “inside-out” hypothesis posits that teeth first evolved inside the mouth and later spread to external armor. The newer “outside-in” hypothesis, supported by Haridy’s findings, flips that idea. It suggests that sensory structures developed in the armor first, then moved inside the mouth as vertebrates evolved jaws and began to feed in new ways.
In both scenarios, nature used the same raw materials—specialized cells that produce dentin—to create tools tailored to new challenges. Whether for feeling water currents or crushing prey, evolution simply repurposed what it already had.
The Ghosts of Teeth Past
We can still see the legacy of this evolutionary story in living animals today. Sharks, skates, and catfish are covered in tooth-like structures called denticles that give their skin a rough, sandpaper-like texture. These are not teeth in the traditional sense, but they contain the same basic tissues—dentin and enamel—and serve similar functions.
Haridy’s lab-raised suckermouth catfish, for example, had denticles connected to nerves—demonstrating that these skin structures weren’t just for protection. They were sensors, too.
“They’re essentially teeth on the outside,” Haridy said.
The similarities between these structures in ancient fish and in modern arthropods suggest something even deeper: that evolution is not always about inventing new things. Sometimes, it’s about reinventing the same thing in new contexts.
A Tangled Lineage Rewritten
The implications of this study go far beyond correcting the identity of a few fossil specimens. It forces a rethinking of how vertebrates evolved—and how researchers identify them in the fossil record.
Some of the Cambrian-period fossils that were once heralded as the earliest vertebrates are now being reclassified as something else entirely. This doesn’t mean the search for the first vertebrate has failed; rather, it’s been refined. The bar has been raised, and the tools for measuring it—synchrotron CT scans, chemical assays, and comparative anatomy—have grown more sophisticated.
“For some of these fossils that were putative early vertebrates, we showed that they’re not,” Shubin said. “But that was a bit of misdirection. We didn’t find the earliest one, but in some ways, we found something way cooler.”
Indeed, uncovering the origins of tooth sensitivity in ancient body armor, and tracing that thread through hundreds of millions of years to the throbbing nerve endings in our own mouths, is no small achievement.
Teeth as Windows into Evolution
At its heart, this study offers a fresh perspective on a tissue we rarely think about unless it hurts. Teeth are not merely tools for chewing or weapons for tearing—they are evolutionary artifacts, born from a sensory need that long predates their culinary use.
They are windows into a distant past where armored fish patrolled the seas and crabs scuttled across Cambrian seafloors, both equipped with finely tuned biological sensors embedded in their hard outer shells.
So the next time your dentist taps a molar or you flinch from a frozen treat, remember: your teeth are more than enamel and pain receptors. They are the descendants of ancient armor, evolved not just for eating, but for feeling the world.
And that sensitivity—annoying as it may be—is a legacy of life’s most ingenious repurposing.
Reference: Yara Haridy, The origin of vertebrate teeth and evolution of sensory exoskeletons, Nature (2025). DOI: 10.1038/s41586-025-08944-w. www.nature.com/articles/s41586-025-08944-w