In the relentless global pursuit of a hydrogen-powered future, the spotlight has long been fixated on platinum (Pt)—the metallic royalty of catalysts. Its unmatched efficiency in accelerating the hydrogen oxidation reaction (HOR), a critical process in hydrogen fuel cells, has cemented its role as a staple in clean energy systems. But with platinum’s price tag climbing ever higher and its natural reserves dwindling, scientists around the world are racing to find alternatives that are not only cheaper but also equally, if not more, effective.
Now, in a breakthrough that could redefine the economics and design of hydrogen energy systems, a team of researchers from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences, in partnership with Anhui University, has unveiled a ruthenium (Ru)-based catalyst so finely tuned and powerfully efficient that it leaves platinum in its wake.
The study, published in the prestigious journal Nano Letters, reveals a radical and elegant approach: fine-tuning the electronic heartbeat of ruthenium atoms through atomic-scale structural engineering. By embedding these atoms within manganese oxide (MnOₓ) lattices, the team has not only overcome a long-standing barrier in Ru catalysis but also opened a new chapter in interfacial catalyst design—offering a 13-fold performance increase over traditional Ru catalysts and an 8-fold improvement over commercial Pt/C catalysts.
Ruthenium’s Double-Edged Potential
Ruthenium isn’t a newcomer to the world of catalysis. It’s long been recognized for its potential as a platinum alternative due to its similar electronic configuration and catalytic behavior. But when it comes to hydrogen oxidation under alkaline conditions, Ru has a glaring weakness: it binds too tightly to intermediates like hydrogen atoms (H*) and hydroxyl groups (OH*), which hinders their release and clogs the reaction pathway.
Think of it like an overly clingy dance partner—great at grabbing onto the rhythm, but bad at letting go when the music changes. This strong adsorption slows down the overall reaction, making Ru an underperformer in practical alkaline fuel cell applications.
So, how do you make ruthenium just reactive enough without becoming overbearing? The answer lies not in changing ruthenium itself, but in redefining the electronic environment it lives in.
Atomic Embedding: Ruthenium Meets Manganese Oxide
Led by Professor Wang Hui, with collaborators Prof. Zheng Fangcai and Prof. Luo Qiquan, the research team designed a novel interfacial architecture by embedding Ru atoms inside the crystal lattice of manganese oxides. This atomic embedding is not merely structural—it is electronic alchemy.
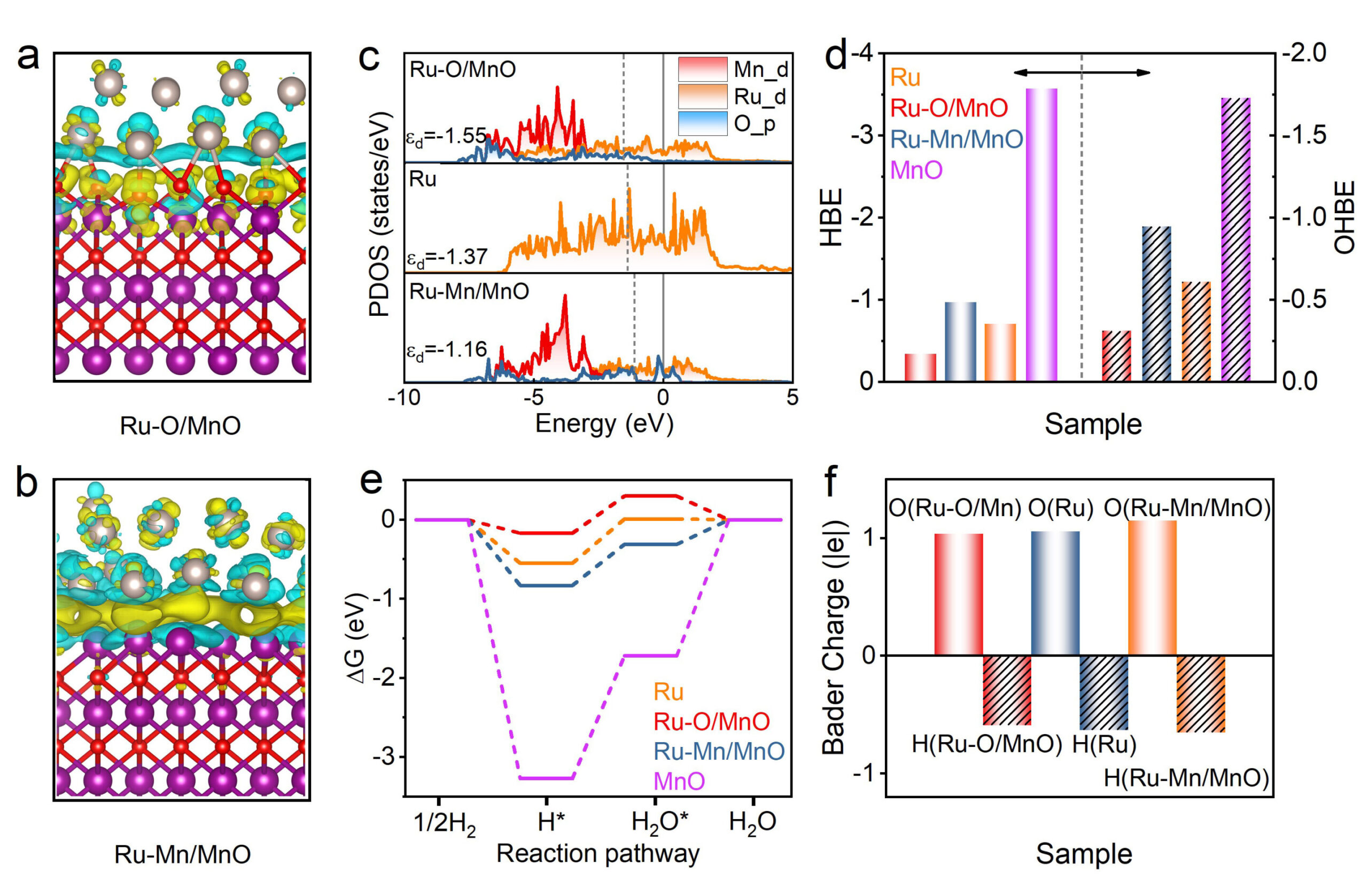
By forming interfacial Ru–O bonds, the embedded ruthenium atoms experience a subtle yet powerful electronic shift. The bonding with oxygen in the manganese oxide matrix induces a downward shift in the d-band center of Ru. In catalysis, the d-band center acts like a tuning dial—it determines how strongly the metal surface binds to reactants and intermediates. A downward shift softens this grip, allowing intermediates like H* and OH* to adsorb and desorb at just the right balance.
In simpler terms, the Ru atoms become more discerning dancers—gracefully engaging with reaction partners without overstepping.
Visualizing the Invisible: Electron Spin Resonance at Work
One of the most compelling aspects of this study is how the researchers confirmed the subtle changes in the Ru environment. They used Electron Spin Resonance (ESR) spectroscopy—a magnetic field-based technique capable of detecting unpaired electron interactions—to observe the fine structure of the Ru–O interface.
These ESR studies were conducted at China’s Steady-State High Magnetic Field Experimental Facility, one of the world’s premier infrastructures for advanced material characterization. ESR allowed the scientists to visualize the magnetic fingerprints of the Ru–O bonds and confirm the successful electronic modulation of Ru atoms.
This marks a significant step forward not only in catalyst design but also in how magnetic field technologies like ESR can be harnessed to validate and guide the development of next-generation catalysts. It is a testament to the synergy between fundamental physics and applied chemistry.
Breaking Barriers: Record-Setting Catalytic Performance
The results were nothing short of astonishing. When tested in a 0.1 M KOH solution—a standard benchmark for evaluating alkaline HOR catalysts—the new Ru catalyst exhibited a mass activity of 1.26 mA per microgram of Ru. That’s 13 times higher than conventional Ru/C catalysts and 8 times better than commercial platinum on carbon (Pt/C).
But performance isn’t just about peak numbers—it’s also about durability and resilience. The embedded Ru catalyst demonstrated:
- Exceptional long-term stability, retaining activity over extended use cycles.
- Strong resistance to CO poisoning, a major Achilles’ heel for many metal catalysts, including platinum.
These attributes are crucial for real-world applications, where fuel cell components are exposed to impurities and continuous operational stress.
Interfacial Engineering: A New Paradigm for Catalyst Design
What makes this study truly pioneering is its strategic shift in catalyst design philosophy. Traditionally, improving catalysts has involved tweaking particle sizes, doping with foreign elements, or supporting metals on conductive substrates. But this research dives deeper—into the very electronic soul of the active site, modifying its reactivity by engineering atomic interfaces.
By anchoring Ru into a MnOₓ lattice, the researchers didn’t just change where the atoms sit—they altered how those atoms think, react, and engage in chemical transformations.
This interfacial electronic engineering is not exclusive to Ru. The principles demonstrated here can be extended to other transition metals and reactions, from oxygen reduction to carbon dioxide reduction. It opens the door to a new breed of tailor-made catalysts designed with quantum-level precision.
Hydrogen’s Horizon: Affordable, Scalable, and Green
The implications of this breakthrough extend far beyond the lab. Hydrogen fuel cells are central to decarbonizing transportation, power grids, and industrial processes. But their widespread adoption has been hampered by high costs and performance bottlenecks—much of it stemming from reliance on platinum.
This Ru-based catalyst changes the equation. Ruthenium is significantly cheaper and more abundant than platinum. With the enhancements demonstrated here, it becomes not only viable but superior in alkaline environments.
Such developments bring us closer to economically scalable hydrogen fuel technologies, enabling more widespread deployment of green energy systems. They also support broader sustainability goals by reducing dependence on rare and geopolitically sensitive materials.
Magnetic Fields and Molecular Futures
Another subtle yet transformative aspect of this work is its integration of ESR spectroscopy as a design and validation tool. Magnetic field technologies, long used in fundamental research, are now showing their power in applied materials science.
The ability to “see” how electrons behave at catalyst interfaces helps researchers make smarter design decisions. It’s like giving chemists a microscope to peer into the minds of atoms—an invaluable asset in an age where nanotechnology and materials innovation go hand in hand.
This sets a precedent for future studies: advanced characterization methods must walk alongside synthesis, guiding each step with precision and insight.
Conclusion: A Catalyst for Change
In a single study, the team from HFIPS and Anhui University has reimagined what’s possible in catalytic science. By embedding ruthenium within a manganese oxide lattice, they have turned a problematic yet promising metal into a superstar catalyst—surpassing platinum in activity, durability, and affordability.
This isn’t just an upgrade in materials—it’s a paradigm shift. A shift that shows how we can design the catalysts of tomorrow by engineering atomic interfaces today.
As the world stands on the cusp of a hydrogen revolution, discoveries like these are more than scientific triumphs—they are technological keystones in the bridge to a sustainable future. A future where clean energy isn’t a luxury, but a standard. And where the quiet dance of atoms, choreographed by brilliant minds, powers the world without polluting it.
Reference: Xiaojuan Zhang et al, Lattice-Confined Ru Electrocatalysts with Optimal Localized Interfacial Electrons for Efficient Alkaline Hydrogen Oxidation, Nano Letters (2025). DOI: 10.1021/acs.nanolett.4c06285