Look around you. Everything you see—the air you breathe, the water you drink, the screen you’re reading this on—is made up of molecules. These molecules, in turn, are built from atoms held together by chemical bonds. But here’s the fascinating part: the way these atoms are arranged in three-dimensional space—their shape—can dramatically affect the properties of the substance they form.
The study of molecular geometry, or the spatial arrangement of atoms within a molecule, may seem abstract at first. After all, molecules are too small to see even with a microscope. But the implications of molecular shapes are anything but abstract. Molecular geometry determines whether a drug molecule fits a receptor in your body, how water interacts with salt, how light reflects off crystals, and even why snowflakes are symmetrical.
In this in-depth yet engaging exploration, we’ll unlock the secrets of molecular geometry—what it is, why it matters, how we predict it, and the elegant rules that govern the shape of everything from water to DNA. We’ll use simple analogies, vivid mental images, and real-world connections to make the invisible world come alive.
Why Molecular Shape Matters
Let’s start with a deceptively simple question: why should we care about the shape of molecules?
Imagine trying to fit a key into a lock. The key’s shape is crucial—too big, and it won’t go in; too small, and it won’t turn. Molecules behave the same way. Their function is often determined by their form. Enzymes, hormones, and pharmaceuticals all rely on their specific geometry to interact with the correct biological targets.
Here are just a few examples of how molecular geometry affects our daily lives:
- Taste and smell: The molecules responsible for flavor and scent only activate our taste buds or olfactory receptors if they have the right shape.
- Medications: The efficacy of a drug often depends on how precisely its molecules fit into target receptors in the body.
- Materials science: The strength, flexibility, and other properties of materials like polymers and crystals are determined by their molecular structures.
- Chemistry of life: The double helix of DNA, the folding of proteins, and the shapes of vitamins and neurotransmitters all derive from molecular geometry.
In short, understanding molecular geometry helps us understand the very nature of matter and life.
What Is Molecular Geometry?
Molecular geometry is the three-dimensional arrangement of atoms in a molecule. It’s determined by the number of atoms, the number of electron pairs, and the type of bonds between atoms (single, double, or triple).
At its core, molecular geometry is a story about balance. Atoms and electrons repel each other—they want to be as far apart as possible. The shape a molecule takes is the most stable one it can find, where these repulsions are minimized.
To predict and describe these shapes, chemists use models and theories—most notably the Valence Shell Electron Pair Repulsion (VSEPR) theory, which we’ll explore in detail shortly.
But before diving into theory, let’s build a strong intuitive understanding of what shapes molecules can take and why.
Atoms, Electrons, and the Push-and-Pull of Geometry
Every atom wants to be “comfortable.” That comfort comes from having a full outer shell of electrons. In molecules, atoms share electrons through chemical bonds to reach that happy state.
But electrons, being negatively charged, repel each other. This repulsion influences where bonds and lone electron pairs can go. Think of each group of electrons as a balloon—they want space, and they push each other away until they’re as far apart as possible.
This is the essence of VSEPR theory: the shape of a molecule is determined by repulsions between electron groups in the valence shell of the central atom.
The VSEPR Theory: Making Geometry Predictable
The Valence Shell Electron Pair Repulsion (VSEPR) model is the most widely used theory for predicting molecular geometry. It’s wonderfully intuitive.
Here’s how it works in a nutshell:
- Electron groups (bonding pairs and lone pairs) around a central atom arrange themselves to minimize repulsion.
- The number of electron groups determines the basic geometry (like linear, trigonal planar, tetrahedral).
- The number of bonding pairs and lone pairs determines the molecular shape (like bent, trigonal pyramidal, etc.).
Let’s take a tour through the most common molecular geometries, using VSEPR as our guide.
Linear Geometry (2 Electron Groups)
Example: Carbon dioxide (CO₂)
- Central atom: Carbon
- Electron groups: 2 bonding pairs (double bonds to oxygen)
- No lone pairs
- Shape: Linear
- Bond angle: 180°
In this geometry, the two electron groups are opposite each other. It’s like two people holding hands and leaning away from each other for maximum space.
Trigonal Planar Geometry (3 Electron Groups)
Example: Boron trifluoride (BF₃)
- Central atom: Boron
- Electron groups: 3 bonding pairs
- No lone pairs
- Shape: Trigonal planar
- Bond angle: 120°
Imagine the arms of a peace symbol or a three-legged stool—all in a flat plane, evenly spaced.
Bent (3 Electron Groups, 1 Lone Pair)
Example: Sulfur dioxide (SO₂)
- Central atom: Sulfur
- Electron groups: 3 (2 bonding pairs, 1 lone pair)
- Shape: Bent or angular
- Bond angle: <120°
The lone pair pushes the bonding pairs closer together, creating a bent shape. It’s like two balloons squeezed by an invisible third balloon.
Tetrahedral Geometry (4 Electron Groups)
Example: Methane (CH₄)
- Central atom: Carbon
- Electron groups: 4 bonding pairs
- No lone pairs
- Shape: Tetrahedral
- Bond angle: 109.5°
This is a key shape in organic chemistry. Picture a three-legged stool with one leg pointing upward—it’s beautifully symmetrical.
Trigonal Pyramidal (4 Electron Groups, 1 Lone Pair)
Example: Ammonia (NH₃)
- Central atom: Nitrogen
- Electron groups: 3 bonding pairs, 1 lone pair
- Shape: Trigonal pyramidal
- Bond angle: ~107°
The lone pair at the top acts like an invisible leg of a tripod, pushing the other three atoms downward.
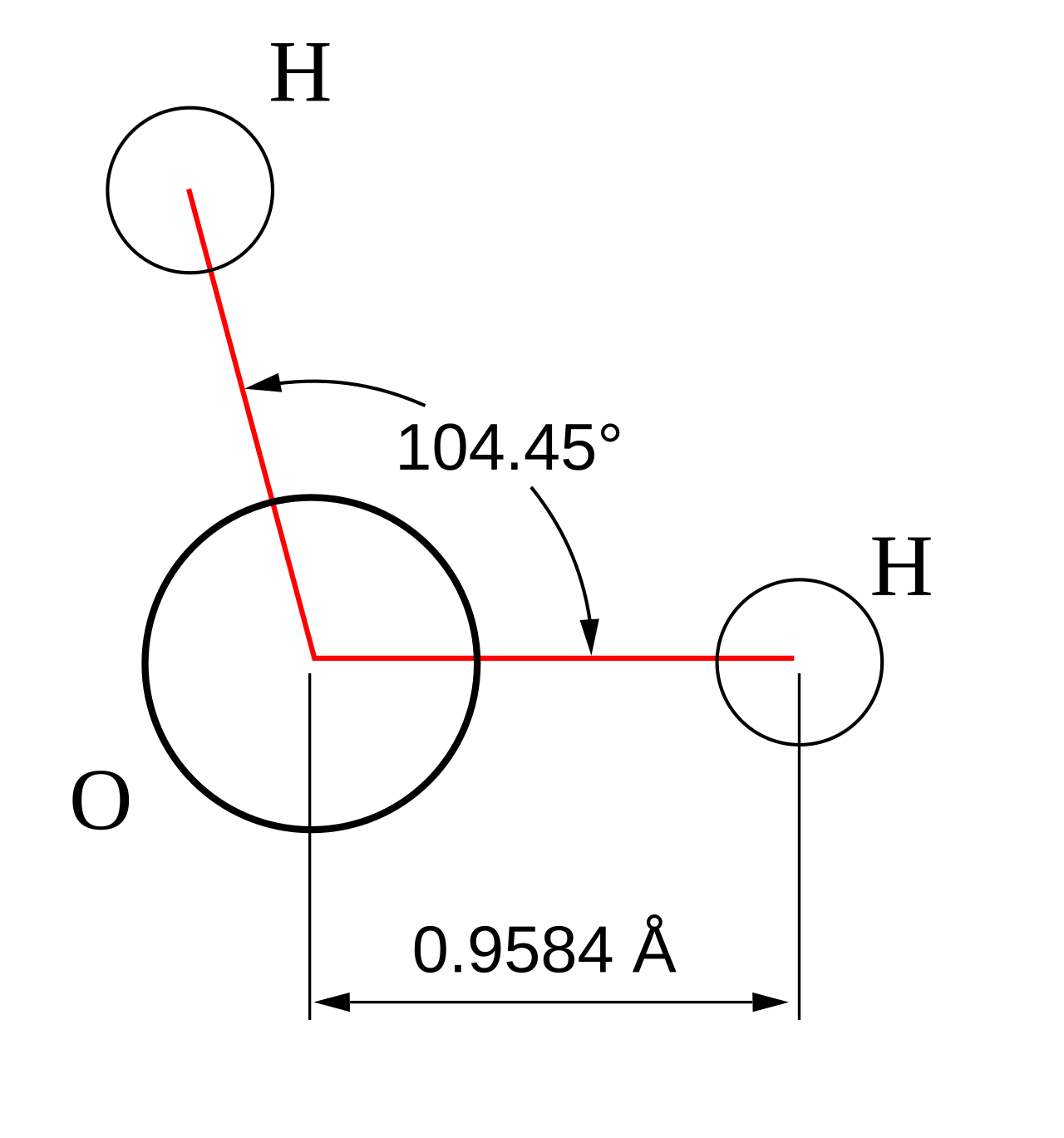
Bent (4 Electron Groups, 2 Lone Pairs)
Example: Water (H₂O)
- Central atom: Oxygen
- Electron groups: 2 bonding pairs, 2 lone pairs
- Shape: Bent
- Bond angle: ~104.5°
This humble molecule is a marvel of geometry. Its bent shape gives water its polarity and incredible solvent properties.
Trigonal Bipyramidal Geometry (5 Electron Groups)
Example: Phosphorus pentachloride (PCl₅)
- Central atom: Phosphorus
- Electron groups: 5 bonding pairs
- Shape: Trigonal bipyramidal
- Bond angles: 90°, 120°
Here, three atoms form a triangle in a plane, and two more stick out above and below. It’s a dynamic, elegant structure.
Octahedral Geometry (6 Electron Groups)
Example: Sulfur hexafluoride (SF₆)
- Central atom: Sulfur
- Electron groups: 6 bonding pairs
- Shape: Octahedral
- Bond angles: 90°
The most symmetrical of all, this geometry looks like two pyramids stuck together base-to-base.
Lone Pairs: The Invisible Shapers
Lone pairs don’t show up as atoms, but they play a huge role in determining molecular geometry. Because they occupy space and repel more strongly than bonding pairs, they compress bond angles and often distort ideal geometries.
Consider the difference between methane (CH₄), ammonia (NH₃), and water (H₂O). All have four electron groups, but lone pairs push the bonding pairs closer together, changing the shape from tetrahedral to trigonal pyramidal to bent.
Polarity: When Shape Meets Electronegativity
Molecular geometry isn’t just academic—it helps determine polarity, which in turn affects boiling point, solubility, and reactivity.
A molecule is polar if:
- It has polar bonds (difference in electronegativity).
- Its shape does not cancel out those dipoles.
For example:
- CO₂ has polar bonds, but it’s linear, so the dipoles cancel out → nonpolar.
- H₂O has polar bonds and a bent shape → polar.
Polarity explains why oil and water don’t mix, why soap works, and why molecules dissolve (or don’t) in different solvents.
Real-World Applications of Molecular Geometry
Let’s bring molecular geometry out of the lab and into the world.
1. Medicinal Chemistry
The shape of a drug molecule determines how well it binds to its target. One mirror-image molecule might relieve pain, while its twin is inactive—or even harmful. This is known as chirality, and it’s a direct consequence of molecular geometry.
2. Materials Science
The hardness of diamonds versus the softness of graphite comes down to geometry. In diamonds, carbon atoms form a tetrahedral lattice; in graphite, they form flat sheets. Same element, different shapes—radically different properties.
3. Climate Science
Molecules like carbon dioxide and methane trap heat because of their shape. Their ability to absorb and re-emit infrared radiation comes from how they vibrate—something dictated by geometry.
4. Food Chemistry
Molecular shape affects flavor. Capsaicin (spicy) and vanillin (sweet) trigger different taste receptors because of their different geometries.
5. Nanotechnology
From DNA origami to self-assembling machines, molecular geometry guides how nanostructures are designed and how they function.
Geometry and Hybridization: How Orbitals Shape the Molecule
We’ve talked about shapes based on electron groups—but what about the orbitals that form bonds? This leads us to hybridization, a concept that explains how atomic orbitals mix to form new shapes suited for bonding.
- sp hybridization → linear geometry
- sp² → trigonal planar
- sp³ → tetrahedral
- sp³d → trigonal bipyramidal
- sp³d² → octahedral
Hybridization gives us another lens to understand why atoms arrange themselves the way they do.
Geometry Beyond Simple Molecules
Larger molecules, like proteins or DNA, have complex geometries governed by similar principles. In proteins, geometry affects folding, which affects function. In DNA, the iconic double helix arises from base-pair geometry and hydrogen bonding.
Even in inorganic chemistry, shapes like square planar or T-shaped arise in coordination compounds. Geometry remains a core concept across all levels of chemistry.
Conclusion: From Simplicity to Complexity
At first glance, molecular geometry might seem like a dry, technical topic. But once you dive in, it becomes clear: it’s the invisible architecture behind everything.
It explains why water flows, why salt dissolves, why DNA holds our code, and why medicines heal. It connects the minuscule scale of atoms to the macroscopic world we experience every day.
Understanding molecular geometry is like learning the grammar of the molecular world. Once you speak its language, everything from hydrogen to hemoglobin makes more sense.
So the next time you drink a glass of water, take a breath, or pop a vitamin—remember that hidden in those simple acts are molecules with complex, elegant shapes, dancing in three-dimensional space, following the timeless laws of molecular geometry.