Imagine waking up one morning and realizing your coffee has lost its aroma, or the scent of fresh flowers no longer reaches you. For many, a diminishing sense of smell might seem like a minor inconvenience, perhaps chalked up to aging or seasonal allergies. But new research suggests it could be something far more profound—a red flag for Alzheimer’s disease long before memory lapses or confusion set in.
Scientists at the German Center for Neurodegenerative Diseases (DZNE) and Ludwig-Maximilians-Universität München (LMU) have uncovered a surprising mechanism that links smell loss to the earliest stages of Alzheimer’s. Their findings, recently published in Nature Communications, point to the brain’s own immune cells mistakenly dismantling critical nerve fibers responsible for odor perception.
The Brain’s Communication Breakdown
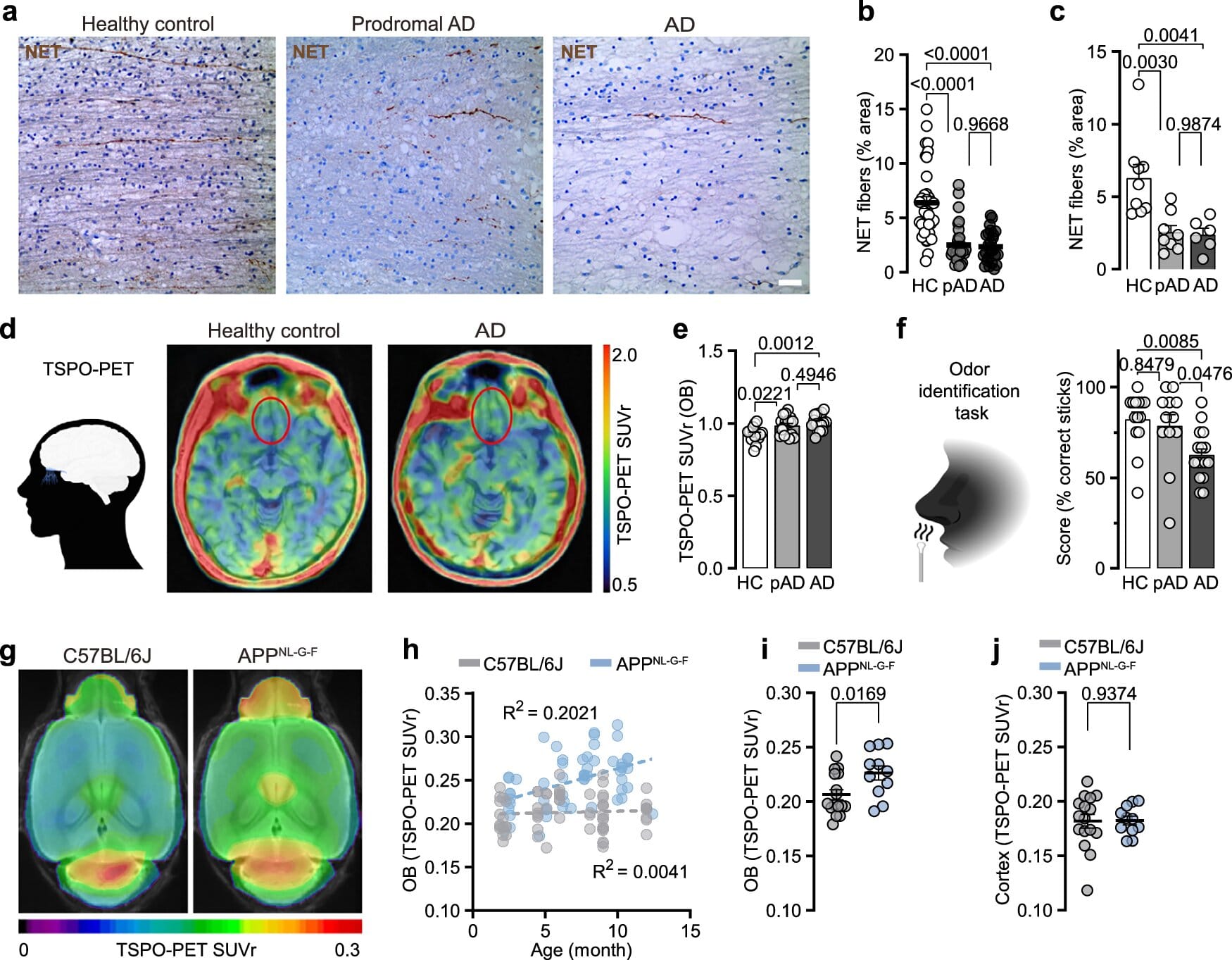
The sense of smell begins in the olfactory bulb, a structure in the forebrain that processes signals from scent receptors in the nose. But the olfactory bulb doesn’t work alone—it relies on input from the locus coeruleus, a tiny but powerful hub in the brainstem that regulates blood flow, sleep cycles, attention, and sensory processing.
The DZNE-LMU team discovered that in early Alzheimer’s, the long nerve fibers that connect the locus coeruleus to the olfactory bulb are among the first casualties. These fibers don’t simply wither away on their own—they are actively targeted by the brain’s immune sentinels, called microglia.
“Normally, microglia serve as guardians, cleaning up defective or unnecessary connections,” explains Dr. Lars Paeger, lead scientist of the study. “But in Alzheimer’s, they misinterpret signals from overactive neurons and break down fibers that are still needed for the sense of smell.”
The “Eat-Me” Signal
At the heart of this immune miscommunication lies a subtle biochemical shift. The researchers found that in affected nerve fibers, a molecule called phosphatidylserine—usually tucked safely inside the cell membrane—flips to the outside.
In biology, this is the equivalent of waving a white flag. Exposed phosphatidylserine acts as an “eat-me” signal to microglia, marking the fibers for removal. This process, known as synaptic pruning, is essential during brain development and in normal life to clear out redundant connections. But in early Alzheimer’s, the pruning seems to go too far, cutting away at the very wiring that allows us to smell.
“We think the hyperactivity of neurons, which is characteristic of Alzheimer’s, triggers this membrane change,” says Paeger. “Once that happens, microglia treat the fibers as defective and eliminate them—even though their loss contributes to dysfunction.”
From Mice to Humans
The conclusions were drawn from a wealth of evidence: genetically engineered mice that develop Alzheimer’s-like features, brain tissue from deceased Alzheimer’s patients, and sophisticated PET scans of living individuals with mild cognitive impairment. Across these models, the same theme emerged—olfactory pathways are disrupted early, and immune-driven pruning plays a central role.
This discovery fills in a major gap. Scientists have long observed that people with Alzheimer’s often lose their sense of smell years before memory problems become obvious. But until now, the biological cause of this phenomenon remained murky.
“Smell deficits in Alzheimer’s have been discussed for decades, but the exact mechanism was unknown,” says Prof. Dr. Jochen Herms, co-author of the study. “Now we see that immune activity, rather than just passive nerve degeneration, is at play—and it begins at a very early stage.”
Why Smell Could Be the Key to Early Diagnosis
The implications of these findings extend far beyond understanding why Alzheimer’s patients lose their sense of smell. They may also hold the key to diagnosing and treating the disease earlier than ever before.
New therapies, including antibodies that target amyloid-beta proteins, are most effective when given before extensive brain damage has occurred. But doctors face a challenge: how to identify patients at risk early enough to make these treatments worthwhile.
This is where smell comes in. If subtle changes in olfactory processing can be reliably linked to early Alzheimer’s activity in the brain, then smell tests—combined with imaging—could become a valuable screening tool.
“Our results suggest that loss of smell is not just a side effect of Alzheimer’s, but a direct result of disease mechanisms,” Herms emphasizes. “This opens the door to using olfactory dysfunction as an early biomarker.”
The Human Side of Science
For patients and families, the research carries both warning and hope. It suggests that something as seemingly mundane as struggling to detect everyday scents could serve as a silent alarm bell, years before dementia symptoms appear. Early recognition could mean earlier treatment, improved outcomes, and more time for families to prepare and adapt.
The study also reminds us that Alzheimer’s is not just a disease of memory. It is a disorder that touches nearly every aspect of brain function, from the way we perceive the world to the way we navigate daily life. A fading sense of smell is not trivial—it is a deeply human loss that disrupts how we connect with food, nature, and even memory itself.
Looking Ahead
While more work is needed to translate these findings into clinical tools, the DZNE-LMU study represents a breakthrough in understanding the hidden beginnings of Alzheimer’s. It shifts the narrative from smell being a quirky symptom to being a meaningful biological clue.
The hope is that one day, a simple smell test could join the arsenal of early screening tools, giving doctors and patients a vital head start against a disease that currently robs millions of memory, independence, and identity.
For now, the message is clear: the nose may know far more about the brain’s health than we ever imagined.
More information: Carolin Meyer et al, Early Locus Coeruleus noradrenergic axon loss drives olfactory dysfunction in Alzheimer’s disease, Nature Communications (2025). DOI: 10.1038/s41467-025-62500-8