For millions of people across the globe, chronic pain is an invisible burden that changes everything. It is not simply a dull ache that lingers, nor is it the kind of temporary discomfort most people know. Chronic pain can last for months, years, or even a lifetime, shaping daily routines, limiting independence, and eroding quality of life. For some, even the touch of clothing, the brush of wind, or the pressure of a handshake can feel unbearable.
Doctors have long known that chronic pain is not just a physical sensation—it is a condition that affects mood, sleep, relationships, and even the ability to work. It is now recognized as one of the leading causes of disability worldwide. Yet, despite decades of research, the exact biological processes that drive this relentless condition have remained elusive. Why do some people’s nerves continue to “scream” long after an injury heals? And why do conventional treatments often fail?
Until recently, the answers remained shrouded in mystery. But a remarkable scientific collaboration may have changed that.
A Genetic Clue Hidden in Plain Sight
An international team of researchers, led by Professor David Bennett of Oxford’s Nuffield Department of Clinical Neurosciences (NDCN) and Professor Simon Newstead from the Department of Biochemistry and the Kavli Institute for NanoScience Discovery, have uncovered something extraordinary: a specific gene linked to chronic pain.
Their study revealed that people carrying a variation of a gene called SLC45A4 were more likely to experience higher levels of pain. This discovery came from analyzing massive genetic datasets, including the UK Biobank and Finland’s FinnGen study, which compare genetic codes with real-world health outcomes.
For years, scientists had theories about molecules known as polyamines—naturally occurring chemicals in the body that play a role in cell function. Too many polyamines, it was thought, might overstimulate pain-sensing neurons, causing them to overfire and send exaggerated pain signals to the brain. But there was a missing piece: no one knew exactly how polyamines moved across nerve cells, or what was responsible for controlling them.
Now, thanks to this breakthrough, that missing piece has been identified. The SLC45A4 gene encodes a neuronal polyamine transporter, essentially the molecular “gate” that regulates polyamine levels in pain-sensitive neurons. For the first time, researchers have pinpointed not only a gene but also its precise function in chronic pain.
From Gene to Structure: Seeing Pain in 3D
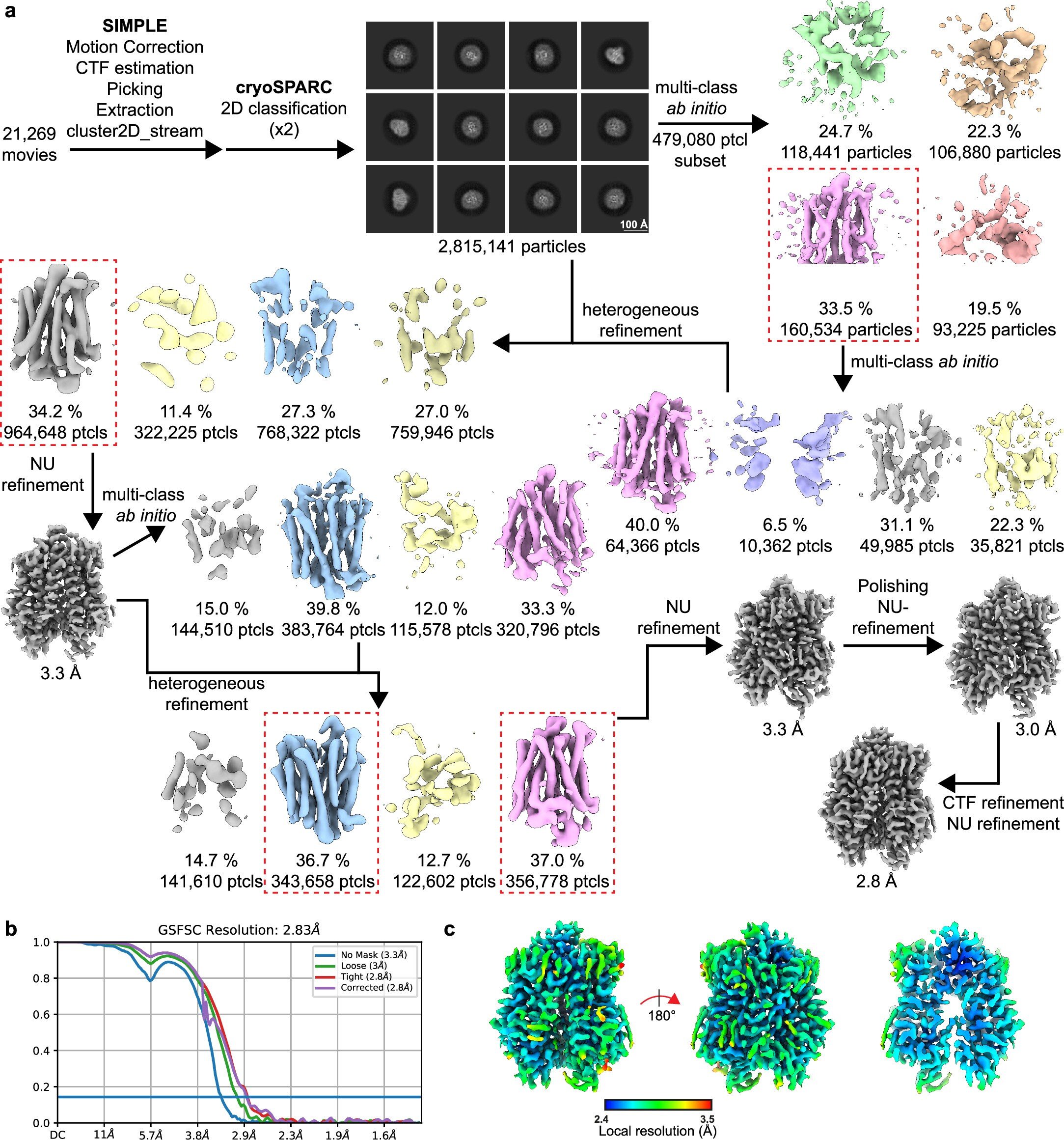
Discovery at the genetic level was only the beginning. To truly understand how SLC45A4 works, the researchers had to see it in action. Using cutting-edge cryo-electron microscopy, Professor Newstead’s group determined the 3D atomic structure of the transporter. This was the first time the protein had ever been visualized in such detail.
Imagine peering inside a lock and seeing exactly how its pins move—that is what the scientists achieved. By mapping the transporter’s shape, they confirmed that it shuttles polyamines across nerve cell membranes, regulating how these cells respond to painful stimuli.
What makes this finding so important is its specificity. Chronic pain has been difficult to treat precisely because scientists lacked a clear target. Most current treatments, such as opioids, act broadly across the nervous system. They dampen pain, yes, but at a devastating cost—addiction, tolerance, and dangerous side effects. Now, with SLC45A4 identified as a key regulator, scientists finally have a targeted pathway to aim for.
Pain Signals and the Nervous System
To appreciate the scale of this discovery, it helps to understand how pain normally works. Pain begins with nociceptors, specialized nerve cells that detect harmful stimuli—like touching a hot stove or cutting your skin. These cells then send warning signals through the spinal cord to the brain, where pain is perceived.
In chronic pain, something goes terribly wrong. Nociceptors become hypersensitive, firing off signals even when there is no real threat. It’s as if the body’s alarm system is stuck in overdrive, constantly ringing.
The new research revealed that SLC45A4 is found in abundance in the dorsal root ganglion—a cluster of sensory neurons that act as a relay point between the body and the brain. Here, the transporter plays a critical role in controlling how much pain information gets transmitted. When scientists tested mice lacking this gene, they found that the animals responded less intensely to pain stimuli, suggesting that SLC45A4 directly influences pain sensitivity.
Moving Beyond Opioids
The implications of this discovery are profound. For decades, the lack of precise treatments for chronic pain has forced doctors to rely on opioids—drugs that blunt pain by flooding the brain with chemicals that affect multiple pathways. While effective in the short term, opioids are notoriously addictive and have fueled a devastating global crisis of dependency and overdose.
The new findings offer hope for a safer future. By developing drugs that specifically target the SLC45A4 transporter, scientists may be able to quiet overactive pain neurons without hijacking the brain’s reward system. This means patients could one day find relief without the risk of addiction.
As Professor Bennett explains, “Chronic pain remains a huge societal problem as it is becoming more common and current treatments fail. We need to understand the mechanisms behind chronic pain in humans and importantly identify new analgesic drug targets.”
This study may represent exactly that.
A Triumph of Collaboration and Discovery
What makes this achievement even more inspiring is how it came together. It was not the work of one lab or one idea, but a true collaboration across disciplines—genetics, neuroscience, biochemistry, and advanced imaging technology.
Dr. Steven Middleton, the study’s lead author, highlighted the excitement of linking SLC45A4 to human pain: “Remarkably, we identified that SLC45A4 is the long-awaited neuronal polyamine transporter, which is particularly important in regulating how some nerves respond to painful stimuli. This has broadened our understanding of pain signaling in the body and opened new avenues of research directed at treating chronic pain.”
The researchers’ efforts are a reminder that scientific progress often depends on patience, persistence, and a willingness to bridge different fields. By combining data science, molecular biology, and neuroscience, they pieced together a puzzle that had baffled medicine for decades.
Looking Toward the Future
While much work remains, the path forward is clearer than ever before. Next steps will involve testing whether drugs can safely target SLC45A4 in humans, reducing chronic pain without harmful side effects. Animal studies offer hope, but translating discoveries into treatments is always a long and careful journey.
Still, the optimism is justified. For the first time, chronic pain researchers have a tangible, testable target. Patients suffering from conditions like neuropathic pain, fibromyalgia, or arthritis could one day benefit from therapies that address the problem at its root.
Professor Newstead summed up the promise best: “Our findings now reveal a new link between membrane transport and chronic pain, paving the way for a deeper understanding of how metabolism and pain are connected in the human body.”
More information: Steven J. Middleton et al, SLC45A4 is a pain gene encoding a neuronal polyamine transporter, Nature (2025). DOI: 10.1038/s41586-025-09326-y