What if your brain had its own internal cleanup crew, poised to spring into action during moments of emotional strain, but then—under prolonged pressure—just walked off the job? A groundbreaking study from researchers at Zhejiang University reveals exactly that. Published in Nature under the title “Stress dynamically modulates neuronal autophagy to gate depression onset,” the study dives deep into the brain’s remarkable capacity to self-regulate—and what happens when that regulation breaks down under chronic stress.
The discovery centers on autophagy, a cellular self-repair process that acts like a molecular recycling system. And the brain region at the heart of the study? The lateral habenula—a tiny but powerful node known to govern mood and motivation, often referred to as the brain’s “disappointment center.”
This study does more than shine a spotlight on an overlooked brain mechanism—it offers a potential leap forward in the treatment of depression. With major depressive disorder affecting over 10.6% of the global population, new insights like these couldn’t come at a more critical time.
The Stress-Autophagy Connection: A Tale of Two Outcomes
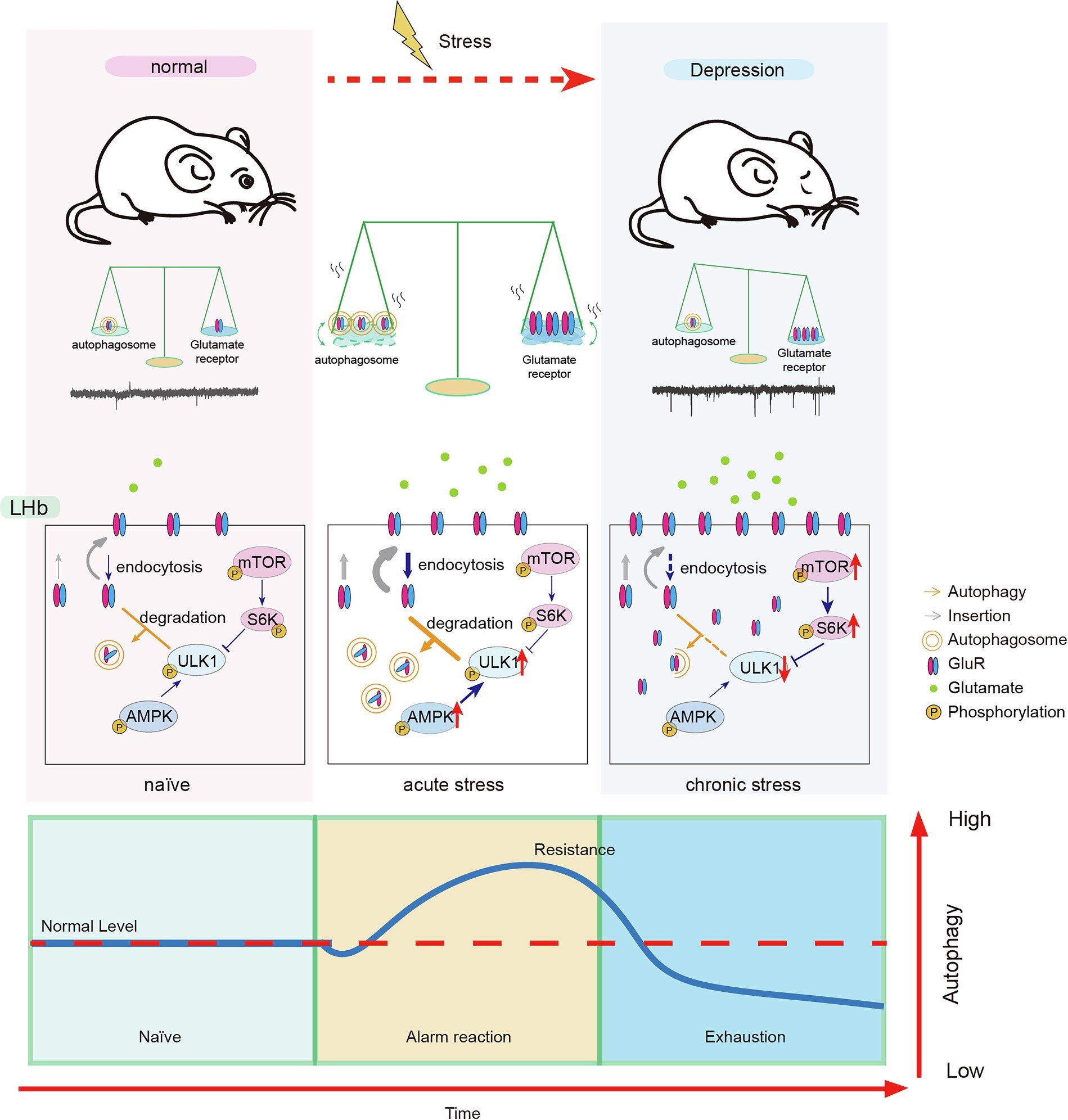
Stress isn’t inherently harmful. In fact, short bursts of stress can activate survival mechanisms, heighten awareness, and even boost mental performance. But the key difference lies in duration. This study showed that acute stress enhances the brain’s internal repair systems—particularly autophagy in the lateral habenula—while chronic stress suppresses them. This distinction is vital for understanding how stress transitions from being adaptive to becoming pathological.
Autophagy is the brain’s internal quality-control mechanism. Think of it as a recycling system that clears out damaged proteins and cellular debris. In neurons, this process is crucial for maintaining healthy synaptic activity and emotional stability.
The researchers discovered that acute stress flips on autophagy, triggering the degradation of overstimulating glutamate receptors and restoring emotional balance. In contrast, chronic stress activates molecular brakes—specifically mTOR signaling—that shut down autophagy, leading to neuronal overexcitation, emotional instability, and behaviors associated with depression.
A Molecular Deep Dive: Experiments in Motion
To unravel these complex dynamics, the team conducted an ambitious series of in vivo and in vitro experiments using mouse models. Mice were subjected to both acute stress (short-term restraint or footshock) and chronic stress (prolonged social defeat or restraint), simulating real-world emotional strain.
Researchers then performed bulk and single-nucleus RNA sequencing, immunostaining, electron microscopy, and Western blot analyses to track autophagic activity across the brain. They also genetically modified mice to knock out key autophagy-related genes—Atg5, Atg7, and Beclin-1—specifically in the lateral habenula, allowing them to trace the direct behavioral effects of autophagy loss.
Their most striking finding? Autophagy was selectively suppressed in the lateral habenula after chronic stress, but enhanced after acute stress. Other emotion-regulating brain regions—including the hippocampus, prefrontal cortex, and ventral tegmental area—showed no such pattern. The lateral habenula emerged as the central hub mediating the stress-autophagy-depression axis.
Antidepressants and Autophagy: A Surprising Overlap
The researchers then asked a pivotal question: Could common antidepressants reverse this autophagy suppression?
They tested a trio of pharmacological heavy-hitters: paroxetine (a classic SSRI), ketamine (a fast-acting NMDA receptor antagonist), and rapamycin (a known autophagy enhancer). Systemic administration of each restored autophagic activity in the lateral habenula and reversed depression-like behaviors in the mice.
The researchers didn’t stop there. They directly infused a Beclin-1 activating peptide into the lateral habenula, and within 30 minutes, previously depressed mice exhibited normal social behaviors and reduced immobility in behavioral despair tests. Even more impressive, these effects lasted up to seven days. When the peptide was administered during ongoing stress, it prevented depression entirely.
In contrast, when autophagy was blocked, even effective antidepressants lost their behavioral impact. This revealed something profound: functional autophagy in the lateral habenula isn’t just a side effect of treatment—it’s a requirement.
Synapses, Glutamate, and On-Demand Cleanup
At the microscopic level, the study revealed that chronic stress caused a buildup of glutamate receptor subunits, leading to heightened excitatory signaling in the lateral habenula—a hallmark of depressive circuitry. By reactivating autophagy, the brain was able to selectively degrade these receptors, restoring balance.
Electrophysiological recordings showed that once autophagy was boosted, neuronal firing patterns normalized and the glutamate surge was subdued. Interestingly, autophagy was especially activated in hyperactive neurons, suggesting it functions as an “on-demand” maintenance crew, stepping in to calm excessive activity before it spirals into dysfunction.
AMPK vs. mTOR: The Battle of the Pathways
At the signaling level, the study identified two opposing molecular forces:
- AMPK signaling, which promotes autophagy, was activated during acute stress.
- mTOR signaling, which inhibits autophagy, dominated during chronic stress.
These two pathways functioned independently of each other, creating a molecular switchboard for how stress is translated into cellular behavior. Understanding how to tip this balance—perhaps with future drug candidates—could hold the key to next-generation antidepressant therapies.
A Paradigm Shift in Depression Treatment?
This study doesn’t just add a new page to neuroscience textbooks—it may rewrite entire chapters. The identification of autophagy in the lateral habenula as a central, causal mechanism in depression offers several compelling implications:
- A Universal Antidepressant Target: Regardless of drug class—SSRI, NMDA antagonist, or mTOR inhibitor—all effective treatments seem to converge on this same autophagic pathway. This could explain why some patients respond to one class of drugs but not another.
- Precision Psychiatry: Measuring autophagy markers in patients (possibly through blood-derived exosomes or imaging biomarkers) could help identify individuals most likely to benefit from autophagy-enhancing therapies.
- Faster-Acting Antidepressants: Unlike conventional SSRIs that take weeks to kick in, interventions targeting autophagy—like the Beclin-1 peptide—showed behavioral effects in under an hour, potentially revolutionizing acute treatment strategies for severe depression and suicidal ideation.
- Preventative Approaches: Administering autophagy-enhancing agents during high-stress periods might prevent the onset of depression, especially in at-risk individuals such as frontline workers, caregivers, or trauma survivors.
The Road Ahead
While the research is still in its preclinical phase, the implications are tantalizing. Next steps will involve translating these findings into human studies, identifying safe and effective autophagy modulators, and exploring the broader role of autophagy in other psychiatric conditions like anxiety, PTSD, and bipolar disorder.
Ultimately, this research reveals the brain as a dynamic, self-healing system—one that can regulate its emotional circuitry at a cellular level when given the right tools. It underscores that depression is not just a chemical imbalance, but a breakdown in a highly regulated stress-adaptation mechanism. And, crucially, it offers hope that with precise intervention, we can help the brain rediscover its own resilience.
References: Liang Yang et al, Stress dynamically modulates neuronal autophagy to gate depression onset, Nature (2025). DOI: 10.1038/s41586-025-08807-4
Alberto Corona et al, Chronic stress drives depression by disrupting cellular housekeeping, Nature (2025). DOI: 10.1038/d41586-025-00910-w