In a groundbreaking scientific achievement that borders on science fiction, an international team of scientists led by Northwestern University has, for the first time ever, directly observed a catalytic chemical reaction in real time—at the atomic level. This unprecedented feat was captured in high-resolution “movies” where individual atoms visibly wriggle, shake, and transform as a chemical reaction unfolds. It’s a moment that shatters long-standing limitations in chemistry and opens a portal into an invisible world that has until now only been theorized.
At the heart of this revolutionary discovery is a technique called single-molecule atomic-resolution time-resolved electron microscopy (SMART-EM). It is, quite literally, a molecular movie camera. And thanks to this technology, scientists have not only recorded atoms in motion during catalysis—they’ve also uncovered entirely new reaction pathways and elusive intermediate molecules that were previously invisible.
This is more than a mere academic achievement—it’s a paradigm shift in chemistry. By finally witnessing catalysis as it happens, researchers are paving the way toward designing more efficient, cleaner, and more sustainable chemical processes for everything from pharmaceuticals to renewable fuels.
A Dream Over 200 Years in the Making
The very idea of seeing a single atom in action was once thought to be the domain of dreamers. Even the most advanced microscopes of the past could only provide snapshots, and only under highly constrained conditions. But with SMART-EM, developed by Professor Eiichi Nakamura and his team at the University of Tokyo, that dream is now a reality.
This isn’t just a microscope—it’s a cinematic window into the molecular world. Nakamura fittingly dubbed the method “cinematic chemistry” because it does more than produce still images. It records frame-by-frame molecular transformations, as if we’re watching a Hollywood film—except the actors are atoms, and the drama unfolds in trillionths of a second.
“Since 2007, physicists have been able to realize a dream over 200 years old—the ability to see an individual atom,” Nakamura said. “But our research group has reached beyond that dream to create videos of molecules to see chemical reactions in unprecedented detail.”
When Chemistry Comes Alive
Led by Northwestern University’s Yosi Kratish and world-renowned chemist Tobin J. Marks, the research team wanted to do something no one had ever done: watch catalysis happen. Catalysts are the invisible workhorses behind everything from manufacturing plastics to powering engines. Yet despite their central role in industry and modern life, the exact atomic-level mechanisms of how catalysts function have remained largely mysterious.
“By visualizing this process and following the reaction mechanisms, we can understand exactly what’s happening in the finest detail,” said Kratish. “Nobody has done this before in catalysis, so I was stunned. When I realized what we accomplished, I had to close my laptop and take a break for a few hours.”
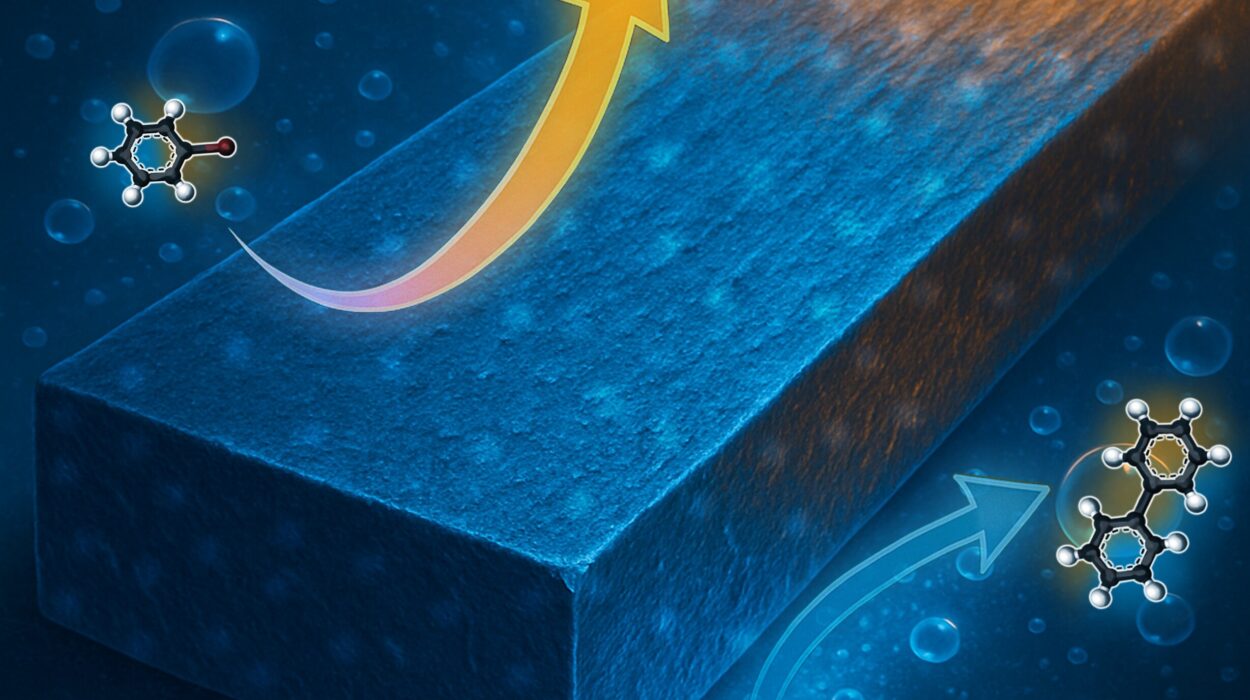
The researchers began with a deceptively simple chemical reaction: the dehydrogenation of ethanol. In this reaction, hydrogen atoms are removed from ethanol (a common alcohol), producing hydrogen gas and aldehyde molecules. It’s a straightforward transformation on paper—but underneath, it hides a tangled web of fleeting molecules and ultra-fast shifts in atomic positions.
In the past, those momentary chemical intermediates would go undetected. They form, change, and disappear faster than any microscope could catch. But SMART-EM changed the game.
Cracking Open the Black Box of Catalysis
A major obstacle in catalysis research is the complexity of heterogeneous catalysts—solid materials that react with gases or liquids on their surfaces. They’re robust, efficient, and used in about 85% of industrial chemical processes. But they’re also messy.
“Heterogeneous catalysts are like black boxes,” said Kratish. “They have an unknown number of sites where reactions can occur. So, we don’t fully understand where and how reactions take place.”
To simplify the problem, the team engineered a single-site heterogeneous catalyst—a rare, well-defined surface where the chemistry could be studied with precision. The catalyst comprised molybdenum oxide particles perched atop a cone-shaped carbon nanotube. This design provided a clean, focused environment where researchers could track reactions with atomic clarity.
Then came the moment of truth: observing the reaction unfold.
Uncovering Hidden Chemistry
What the scientists discovered was far from expected. Conventional wisdom held that ethanol hits the catalyst, loses hydrogen, and forms an aldehyde—which then simply floats away. But the SMART-EM footage revealed something else entirely.
The aldehyde didn’t just disappear. Instead, it stuck around on the catalyst’s surface. There, it linked with other aldehyde molecules, forming short-chain polymers—an unanticipated intermediate step. Even more surprising was the formation of hemiacetal molecules, created when alcohol and aldehyde combined in a side reaction.
These fleeting intermediates, once invisible and theoretical, were now clearly seen. And they weren’t just scientific curiosities—they were critical to understanding how the reaction truly unfolded.
To corroborate their discovery, the team deployed a battery of scientific tools—X-ray analysis, theoretical modeling, computational chemistry, and other forms of microscopy. The data converged: the SMART-EM observations were spot-on.
“This is a big breakthrough,” Kratish said. “SMART-EM is changing the way we look at chemistry. Eventually, we want to isolate those intermediates, control the amount of energy we put into the system, and study the kinetics of a live organic catalytic transformation. That will be phenomenal. This is just the beginning.”
A Technological Triumph
One of the central challenges the team overcame was the fragility of organic molecules. Traditional transmission electron microscopes use high-energy electron beams that easily destroy soft, carbon-based matter long before meaningful data can be recorded.
But SMART-EM was designed to tread gently. By using ultra-low doses of electrons, it captures images without obliterating its subject. Then, by compiling these frames into rapid sequences, it delivers dynamic visualizations of processes that once played out in total secrecy.
“Most conventional TEM techniques operate at conditions that easily damage organic molecules,” Kratish explained. “This makes it extremely challenging to directly observe sensitive catalysts or organic matter during a reaction.”
With SMART-EM, the impossible becomes possible. Atoms are no longer static dots on a screen—they’re dynamic players in an elaborate molecular ballet.
Implications for a Cleaner, More Efficient Future
Why does this matter beyond the lab?
Because catalysts make modern life possible. They’re used to create fuels, fertilizers, plastics, pharmaceuticals, and more. If we can understand how catalysts work—truly work, down to the individual atoms—we can make them better. That means greener manufacturing, cheaper medicines, and clean energy breakthroughs like efficient hydrogen fuel production.
“To make chemical processes more efficient and environmentally friendly, we need to understand exactly how catalysts work at the atomic level,” said Tobin J. Marks, senior author of the study. “Our study is a big step toward achieving that.”
Marks is one of the world’s foremost experts in catalysis and holds multiple professorships across chemistry and engineering. Alongside Kratish, he led a collaboration that included Northwestern’s Michael Bedzyk and George Schatz, and the University of Tokyo’s Eiichi Nakamura and Takayuki Nakamuro—the pioneers behind SMART-EM.
The Beginning of a New Era in Chemistry
The study, published in the journal Chem, is more than a scientific paper—it’s a marker of a new epoch in molecular science. With SMART-EM, chemists are no longer fumbling in the dark, making educated guesses about what happens during a reaction. Now, they can see it. In exquisite, atomic detail.
And the implications go far beyond ethanol. The same approach could be applied to countless other reactions—especially those that are too fast, too subtle, or too complex to be studied by conventional methods.
In a sense, chemistry is entering its own version of the motion picture era. Where once we had only stills, now we have dynamic storytelling—reactions unfolding in real time, revealing surprises, twists, and insights that textbooks never anticipated.
This is the new face of chemistry. And it moves.
Reference: Yosi Kratish et al, Atomic-resolution imaging as a mechanistic tool for studying single-site heterogeneous catalysis, Chem (2025). DOI: 10.1016/j.chempr.2025.102541