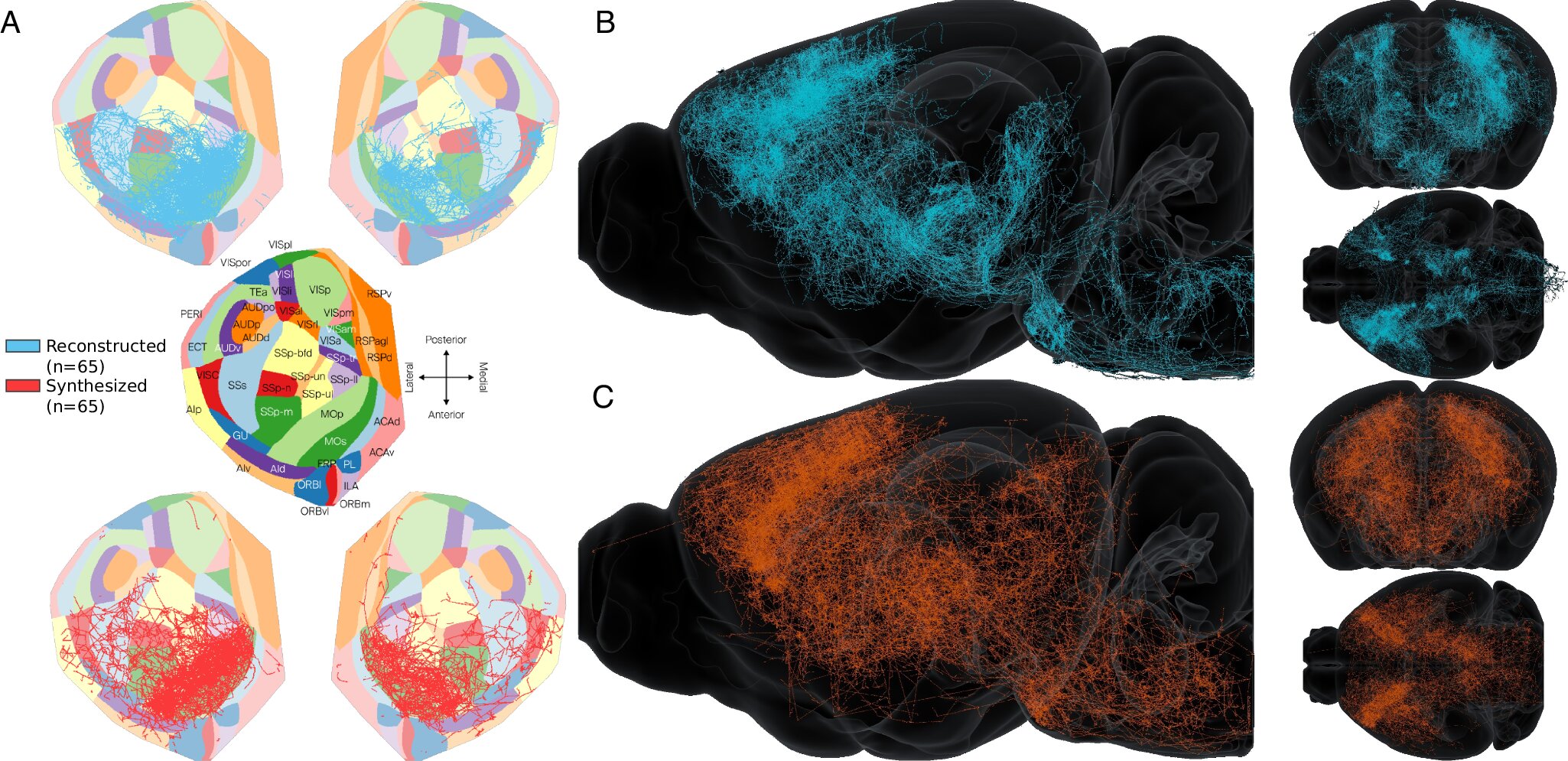
The human brain is often described as the most complex structure in the known universe. With billions of neurons and trillions of connections, it is a living network that gives rise to thought, memory, imagination, and identity itself. For decades, scientists have dreamed of creating a complete map of these connections—a “connectome”—that would reveal how the brain truly works. But despite incredible advances in imaging and data collection, much of this vast neural web has remained hidden.
Now, researchers at EPFL’s Blue Brain Project in Switzerland have unveiled a breakthrough method that could change this picture. By combining biological data with mathematical and computational models, they have created brain-wide, biologically realistic digital maps of the mouse brain’s wiring. Their work, published in Nature Communications, represents a powerful step toward building comprehensive connectomes that could transform neuroscience and medicine.
The Challenge of Mapping the Mind
One of neuroscience’s greatest challenges has always been scale. Imaging technologies can capture intricate details of neurons and their branches, but only within small regions. Scaling this up to the whole brain has proven daunting. While experimental datasets are expanding, they remain too sparse to capture every connection—especially those linking distant brain regions. Yet these long-range pathways are often the key to understanding higher cognitive functions and the roots of neurological diseases.
Without complete wiring maps, scientists face major blind spots. How does information travel across the brain to create complex thought? Why do certain networks break down in disorders like Alzheimer’s or schizophrenia? These questions cannot be fully answered without detailed connectomes.
From Biology to Digital Reality
The team, led by Professor Henry Markram and spearheaded by neuroscientist Lida Kanari, took a novel approach. Instead of relying solely on physical reconstructions of neurons, they turned to computational modeling to fill in the missing pieces.
Their method begins with large datasets of “axonal reconstructions”—tracings of how neurons extend their long, threadlike projections (axons) through the brain. By analyzing these data with machine learning, the researchers were able to group neurons by their wiring patterns.
Then, building on a mathematical model first developed in 2022, first author Rémy Petkantchin designed a computational method to generate synthetic axons. These digital axons mimic real ones: they branch, curve, and connect in the same way their biological counterparts do, even replicating the subtle differences between local and long-distance wiring.
Creating a Digital Connectome
By generating thousands of these synthetic axons, the team constructed a complete digital model of the mouse brain’s wiring. Crucially, the resulting connectome was not just a random simulation. It reflected the same patterns found in experimental data, capturing the delicate architecture of neural networks and preserving critical long-range connections that are often missing in partial datasets.
This synthetic connectome is more than just a digital replica—it is a powerful tool. It allows scientists to fill gaps in experimental maps, test theories about brain organization, and even simulate how diseases disrupt neural wiring. In essence, it creates a digital playground for neuroscience, where questions too complex—or impossible—to answer in living animals can be explored.
A Gateway to Medical Breakthroughs
The implications of this research reach far beyond academic curiosity. A detailed, biologically realistic connectome can transform how we approach brain disorders. Neurological and psychiatric conditions often involve disrupted connectivity: autism, epilepsy, depression, and dementia all leave distinct signatures in the brain’s wiring. With synthetic connectomes, researchers can model these disruptions and test potential interventions in a virtual environment before moving to clinical trials.
Furthermore, digital brain maps can guide new experiments, helping scientists focus on the most promising regions and pathways. They can also power large-scale brain simulations, advancing our understanding of how patterns of activity give rise to behavior and thought.
From Mice to Humans
While this study focused on the mouse brain, its impact may be just the beginning. The principles behind the method are not species-specific. As more biological data becomes available, the same approach could be applied to larger and more complex brains—including humans.
Imagine, one day, having a biologically realistic digital twin of the human brain. Such a model could revolutionize personalized medicine, allowing doctors to test treatments on a patient’s digital brain before applying them in real life. It could also deepen our understanding of consciousness itself, bringing us closer to answering some of the most profound questions in science and philosophy.
The Dawn of a New Era in Neuroscience
Science has always advanced by bridging gaps—between observation and theory, between biology and mathematics, between imagination and evidence. The work of the EPFL team embodies this spirit. By weaving together biological data and computational models, they are not just mapping neurons; they are building a new way of seeing the brain.
The journey to a complete connectome is far from over. But with each step, we come closer to unraveling the mysteries of the mind. The digital mouse brain created in this study is not just a model—it is a promise of what’s possible when human curiosity, advanced technology, and scientific rigor come together.
We are, in many ways, at the dawn of a new era in neuroscience. A time when the brain’s hidden wiring is no longer a mystery but a map—one that could guide us toward cures, insights, and a deeper understanding of what it means to be human.
More information: Remy Petkantchin et al, Generating brain-wide connectome using synthetic axonal morphologies, Nature Communications (2025). DOI: 10.1038/s41467-025-62030-3