Pain, despite its unpleasantness, is one of the body’s most essential warning systems. It’s the body’s way of shouting, “Stop! Something’s wrong.” When you touch a hot stove, stub your toe, or accidentally hit your head, that jolt of pain is your nervous system protecting you from harm. This is acute pain — sharp, temporary, and instructive. It teaches avoidance and promotes healing. In this sense, pain is not an enemy but a survival ally.
But for millions around the world, this alarm never turns off. Long after a wound heals or an injury fades, the pain remains — relentless, invisible, and isolating. This is chronic pain, a condition that affects nearly 50 million people in the United States alone. For these individuals, the body’s warning signal becomes a constant scream.
“It’s not just an injury that won’t heal,” explains neuroscientist J. Nicholas Betley of the University of Pennsylvania. “It’s a brain input that’s become sensitized and hyperactive.” In other words, the pain may not always be in the body — it may live in the brain itself.
The Brain’s Hidden Pain Switch
In groundbreaking research led by Betley and his collaborators at the University of Pittsburgh and Scripps Research Institute, scientists have uncovered a crucial piece of the puzzle — a specific group of brain cells that may control long-term pain.
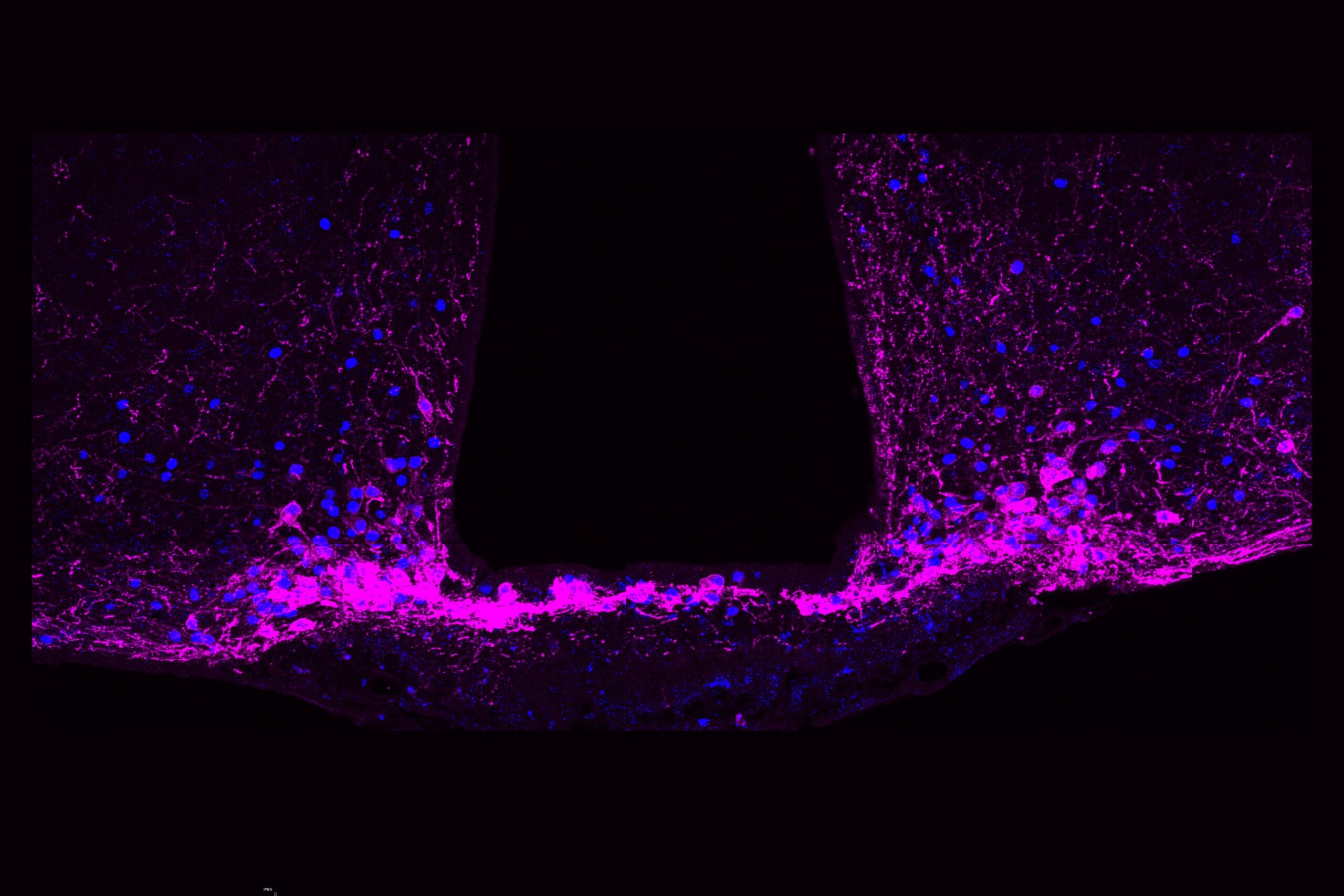
These cells, known as Y1 receptor (Y1R)-expressing neurons, reside in a part of the brainstem called the lateral parabrachial nucleus (lPBN). This tiny region acts as a hub where the brain processes pain, hunger, thirst, and fear — all fundamental survival drives.
What makes these neurons so fascinating is that they seem to function like a biological “switchboard.” They help the brain decide which signals deserve attention. Should the body focus on hunger, or pain? On fear, or thirst? When danger or urgent need arises, the brain can, astonishingly, quiet the pain signal to prioritize survival.
The research, published in Nature, suggests that there is a built-in mechanism in the brain capable of turning down pain’s volume. “There are circuits in the brain that can reduce the activity of neurons that transmit the signal of pain,” the scientists report.
This discovery reframes how we understand chronic pain — not merely as a persistent signal from an injured body part, but as an ongoing miscommunication within the brain’s internal circuits.
Watching Pain in Real Time
To track these elusive neurons, the researchers used calcium imaging, a powerful technique that allows them to watch neurons fire in real time in animal models. The patterns were astonishing.
In response to acute pain, the Y1R neurons lit up briefly — like a spark. But during chronic pain, they stayed active, continuously firing in what neuroscientists call a “tonic” state. This steady hum of neural activity was like an idling engine — the pain persisted even when the external source of injury was gone.
Betley compares it to a car that keeps rumbling long after the key is removed from the ignition. “The signal of pain keeps running,” he explains, “even when it shouldn’t.” This ongoing neural rhythm may be what encodes the enduring sensation of pain that patients feel for months or even years after an injury heals.
Hunger, Fear, and the Power to Silence Pain
The path to this discovery began in 2015, when Betley noticed something curious: hunger seemed to dampen long-term pain responses.
“When you’re really hungry, you’ll do almost anything to get food,” he says. “It felt like hunger was more powerful than Advil at reducing pain.”
That simple observation led to a revolutionary idea — that the brain has a built-in hierarchy of needs, and when survival is at stake, it can suppress pain to focus on what’s more urgent.
Further experiments led by Betley’s graduate student, Nitsan Goldstein, revealed that other powerful instincts — such as thirst and fear — also reduced chronic pain responses. This suggested that the brain uses a kind of “priority filter” at the level of the parabrachial nucleus, blocking long-lasting pain when other life-preserving demands take over.
Goldstein explains it beautifully: “If you’re starving or facing a predator, you can’t afford to be overwhelmed by lingering pain. The brain releases chemicals that temporarily silence that signal so you can survive.”
Neuropeptide Y: The Chemical Messenger of Relief
At the center of this mechanism is a molecule called neuropeptide Y (NPY). This small but mighty neurotransmitter helps the brain manage conflicting needs — hunger, fear, and pain. When survival instincts dominate, NPY binds to Y1 receptors in the parabrachial nucleus, effectively dampening ongoing pain signals.
Goldstein likens it to an “override switch.” When the body is starving or in danger, the brain flips that switch, and pain fades into the background. It’s not that the pain disappears — it’s that the brain temporarily reorders its priorities.
This discovery reveals something profound about human biology: pain, though deeply personal and emotional, is also deeply programmable. The same brain that amplifies pain can also quiet it when it must.
A Mosaic of Pain and Possibility
When the researchers examined the structure of the Y1R neurons more closely, they found something unexpected — these neurons weren’t neatly clustered together. Instead, they were scattered across many different cell types, forming a mosaic pattern throughout the brainstem.
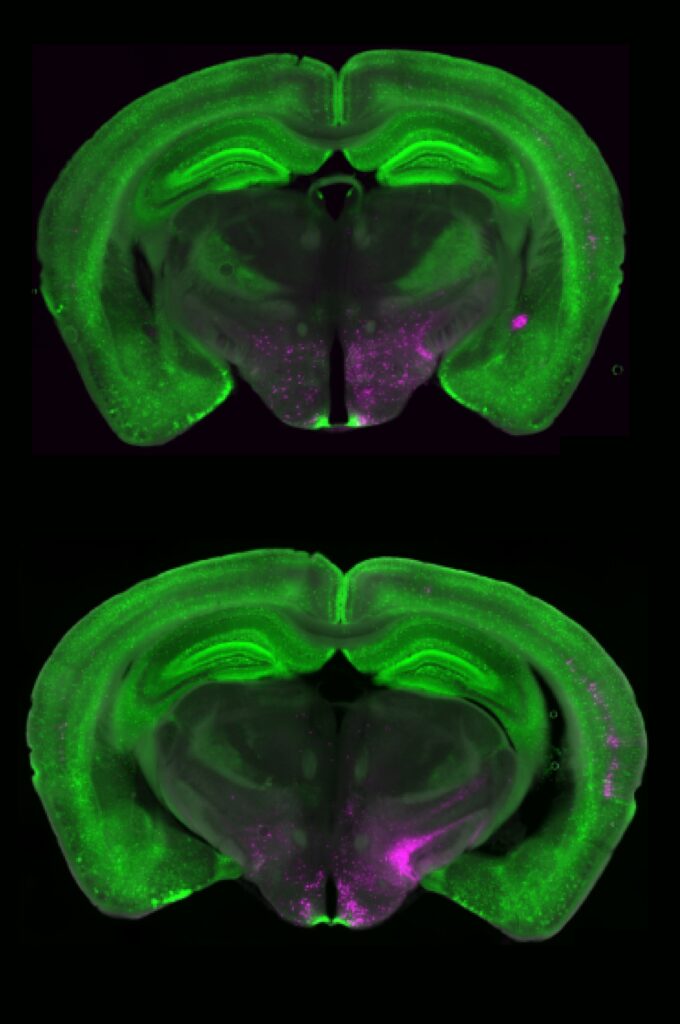
“It’s like looking at cars in a parking lot,” Betley explains. “We expected all the Y1R neurons to be a cluster of yellow cars parked together, but they were like yellow paint splattered across red, blue, and green cars.”
This scattered organization may give the brain remarkable flexibility. It means that pain can be modulated across multiple circuits simultaneously, depending on context — whether the pain is physical, emotional, or environmental.
Rethinking Pain Treatment
The implications of this discovery are enormous. Chronic pain is one of medicine’s most challenging mysteries — often invisible, hard to diagnose, and resistant to treatment. Many patients live with pain that has no clear source or injury.
“What we’re showing,” says Betley, “is that the problem may not be in the nerves at the site of injury, but in the brain circuit itself.”
That insight opens an entirely new frontier in treatment. Instead of targeting the body’s nerves with drugs or surgery, scientists may one day be able to target these brain circuits directly — restoring balance to neurons that have become hyperactive.
Moreover, Betley believes that these Y1R neurons could serve as biomarkers — measurable indicators of chronic pain. For the first time, doctors could use brain imaging to identify and monitor persistent pain states, rather than relying solely on a patient’s description of their suffering.
Behavior, Mind, and the Modulation of Pain
Perhaps the most hopeful aspect of this research is that these pain circuits are not fixed — they are flexible. If hunger and fear can naturally suppress chronic pain, then behavioral interventions that tap into similar neural pathways may also help.
Exercise, meditation, mindfulness, and cognitive behavioral therapy — all of which have been shown to influence brain activity — might literally “train” the brain to quiet overactive pain circuits.
“The future isn’t just about designing a pill,” Betley emphasizes. “It’s about understanding how behavior, training, and lifestyle can change the way these neurons encode pain.”
This suggests a holistic new era in pain medicine — one where biology, psychology, and behavior work together to heal both body and mind.
The Future of Pain Science
The discovery of Y1R neurons and their connection to survival instincts redefines what it means to live with pain. It suggests that our brains are not passive victims of suffering — they are active regulators, capable of adaptation and recalibration.
For millions who endure chronic pain, this science offers a glimmer of hope: that the same brain which traps them in pain may also hold the key to freedom from it.
Pain, after all, is both a teacher and a storyteller. It warns, it protects, it reminds us we are alive. But sometimes, the story needs to change — from endless alarm to gentle awareness, from suffering to understanding.
And in that transformation, the brain — mysterious, adaptable, and compassionate — may be our greatest healer.
More information: Nicholas Betley, A parabrachial hub for need-state control of enduring pain, Nature (2025). DOI: 10.1038/s41586-025-09602-x. www.nature.com/articles/s41586-025-09602-x