Inside the human body, millions of nerve fibers carry electrical messages that control everything from movement to memory, emotion, and vision. These signals travel at lightning speed thanks to the myelin sheath — a thin layer of protective insulation that wraps around the nerves, allowing smooth and rapid communication between the brain and body.
In people living with multiple sclerosis (MS), this elegant communication system begins to break down. The immune system, designed to defend the body, mistakenly turns against it. It attacks the myelin sheath, leaving the nerves exposed and vulnerable. Signals that were once seamless become erratic or blocked entirely, resulting in symptoms like numbness, tingling, blurred vision, fatigue, and even paralysis.
More than 2.9 million people worldwide are affected by MS, and while modern medicine has made enormous strides in slowing the disease’s progression, there remains one crucial frontier yet to be conquered — the ability to repair what’s been lost.
The Need for True Healing
Current treatments for MS primarily focus on reducing inflammation and preventing new immune attacks. These therapies can slow disease progression and lessen relapses, but they do not repair damaged nerves or restore lost myelin. Once the myelin sheath is stripped away and neurons are damaged, the brain’s communication network can falter permanently.
Scientists have long sought a way to reverse this process — to coax the nervous system into healing itself. A treatment that could restore myelin wouldn’t just slow MS; it could potentially help people regain lost function, independence, and quality of life.
Now, in a groundbreaking step forward, researchers from the University of California, Riverside (UCR) and the University of Illinois Urbana-Champaign (UIUC) have discovered promising compounds that might finally make this dream a reality.
The Discovery That Sparked Hope
The research, recently published in Scientific Reports, represents more than a decade of collaboration between Seema Tiwari-Woodruff, a professor of biomedical sciences at UCR, and John Katzenellenbogen, a professor of chemistry at UIUC. Together, their teams have been pursuing one of the most challenging goals in neuroscience: finding a compound capable of rebuilding myelin.
The journey began with a molecule known as indazole chloride. In earlier studies, indazole chloride showed potential to promote remyelination — the process of regenerating the myelin sheath — while also modulating the immune system. It worked well in laboratory models of MS, but it had major drawbacks: it wasn’t safe enough, and it didn’t have the properties needed to become a viable human drug.
Rather than abandon the idea, the researchers took it as a challenge. They began redesigning the molecule, tweaking its structure, and testing new versions to find one that could perform better in the human body. This massive effort led them to screen more than 60 indazole chloride analogs — chemical relatives of the original compound.
From this intense search emerged two star candidates: K102 and K110.
K102: A New Light for Myelin Repair
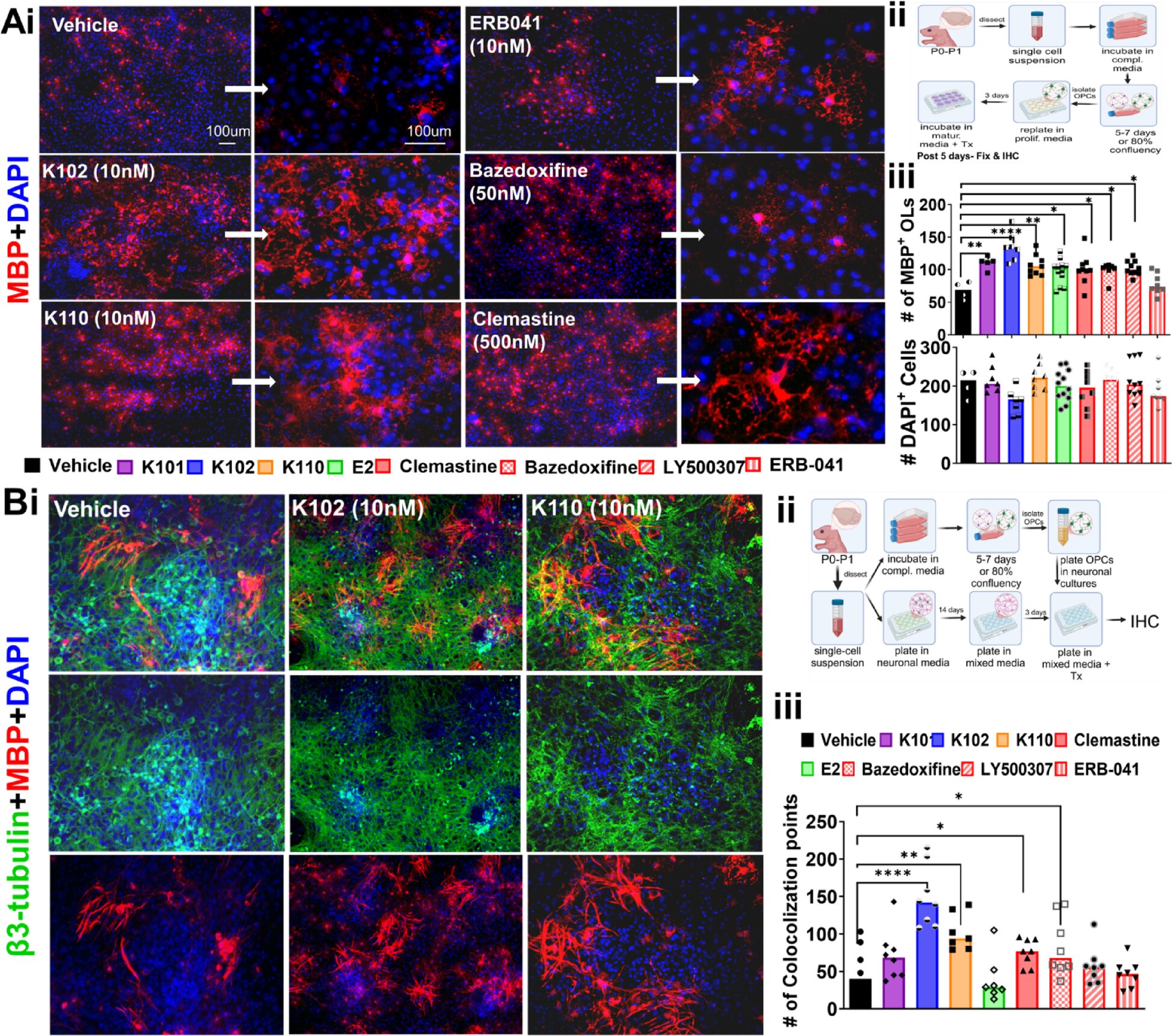
Among the compounds tested, K102 stood out as the most promising. In both animal models and human cell studies, K102 not only encouraged the repair of damaged myelin but also helped regulate the immune system — a critical balance in treating autoimmune diseases like MS.
What makes this discovery especially exciting is its performance in human-derived cells. The researchers tested K102 on oligodendrocytes — specialized cells in the central nervous system responsible for producing myelin. These cells were generated from induced pluripotent stem cells, essentially reprogrammed human cells capable of becoming various tissue types.
In these experiments, K102 boosted the maturation of oligodendrocyte precursor cells, enabling them to form new myelin layers around nerve fibers. This result suggests that the therapy may work not just in mice, but in humans — a rare and hopeful sign in the long, uncertain road of drug development.
If successful, K102 could help restore nerve conduction, reduce disability, and perhaps even allow people with MS to regain functions once thought lost forever.
K110: A Partner in the Pipeline
The second compound, K110, has its own promise. While it works differently in the brain and spinal cord, early data suggest it may be useful for other forms of nerve injury — including spinal cord trauma or traumatic brain injury. The research team believes both compounds could eventually lead to a new class of regenerative therapies targeting diseases marked by demyelination or neuronal damage.
By keeping K110 in the pipeline, the scientists have opened multiple potential paths for healing, expanding beyond MS to conditions that currently have few treatment options.
A Collaboration Years in the Making
The story of this discovery is also a story of perseverance and partnership. For more than 12 years, Tiwari-Woodruff and Katzenellenbogen have worked together, combining the worlds of biomedical science and chemistry. Their shared goal: to bridge the gap between lab research and real-world medicine.
In a statement, Tiwari-Woodruff reflected on this journey: “Our work represents more than a decade of collaboration, with the last four years focused on identifying and optimizing new drug candidates that show strong potential to treat MS and possibly other neurological diseases involving demyelination.”
Their research eventually led to a partnership with Cadenza Bio, Inc., a biotechnology company now responsible for advancing K102 into the next phase of testing. The company has licensed the program and is moving forward with the non-clinical studies needed to begin human trials.
It’s a long process, filled with regulatory hurdles and complex testing requirements, but the scientists remain hopeful. As Tiwari-Woodruff put it, “It’s been a long journey — but this is what translational science is all about: turning discovery into real-world impact.”
From Laboratory Discovery to Human Hope
Drug development is notoriously slow and challenging. Many promising compounds never make it past the lab bench. Yet, the path taken by K102 and K110 reflects how scientific discovery evolves — one step, one experiment, and one insight at a time.
What makes this research remarkable is its focus on remyelination, a process that could shift the treatment paradigm for MS and similar diseases. Rather than only preventing immune attacks, this approach aims to restore what has been lost — helping the nervous system heal itself.
For millions of people living with MS, that shift could mean the difference between slowing decline and reclaiming vitality.
The Science Behind the Hope
To understand the potential impact of K102, it helps to look at how myelin repair works. Under normal conditions, when the myelin sheath is damaged — as might happen during infection or inflammation — the body activates a repair process. Immature cells called oligodendrocyte precursor cells travel to the site of injury, mature into myelin-producing cells, and rebuild the insulation around the nerve fibers.
In MS, however, this natural repair system fails. Chronic inflammation and immune attacks overwhelm the regenerative cells, leaving nerve fibers exposed and vulnerable to degeneration. Over time, this leads to irreversible disability.
K102 appears to support both sides of this equation — reducing inflammation and stimulating repair. It encourages oligodendrocytes to mature and wrap axons with fresh myelin, while also calming the immune response that causes the initial damage. This dual action gives it a unique position among potential MS treatments.
Looking Toward the Future
Cadenza Bio is now working through the necessary stages before clinical testing in humans can begin. This includes toxicity testing, formulation studies, and the creation of manufacturing protocols that meet strict medical standards. While clinical trials may still be some time away, the early signs are inspiring.
The implications reach beyond MS. If these compounds can effectively promote remyelination in humans, they could also help treat a range of neurological conditions — from spinal cord injuries to stroke and neurodegenerative diseases like Parkinson’s or Alzheimer’s.
The potential ripple effect of this research is vast. Each step forward in understanding myelin repair could transform how medicine approaches brain and nerve health for decades to come.
More information: Chloroindazole based estrogen receptor β ligands with favorable pharmacokinetics promote functional remyelination and visual recovery, Scientific Reports (2025). DOI: 10.1038/s41598-025-20254-9