For centuries, nature has shown us a fascinating asymmetry — from the coiling of a snail’s shell to the structure of our own DNA. Now, scientists have stepped into that mirror world, quite literally. For the first time, researchers have created and studied a fully functional mirror-image nanopore — a molecular gateway built entirely from D-amino acids, the right-handed versions of life’s usual left-handed building blocks.
This breakthrough, led by Prof. Dr. Kozhinjampara R. Mahendran at the Rajiv Gandhi Center for Biotechnology (India), in collaboration with Constructor University (Germany) and other international partners, marks a defining moment in nanoscience. Published in Nature Communications, the discovery not only challenges our understanding of molecular symmetry but also opens a door to potential biomedical revolutions, including selective cancer treatments.
The Secret Language of Life’s Handedness
Every protein in your body, every enzyme that helps you breathe, move, or think, is made of L-amino acids — molecules that twist to the left. Their mirror images, D-amino acids, exist too, but in nature, they play only small, supporting roles. It’s as if life has always written its code with the left hand, leaving the right hand mostly idle.
Constructing an entire protein — not just a fragment — from these D-amino acids is extraordinarily difficult. It’s like recreating a left-handed glove perfectly fitted for the right hand, but on a molecular scale. Yet, doing so comes with extraordinary advantages. D-proteins are more resistant to degradation, meaning they can survive longer in biological environments and resist enzymatic breakdown — a key property for creating durable drugs or molecular tools.
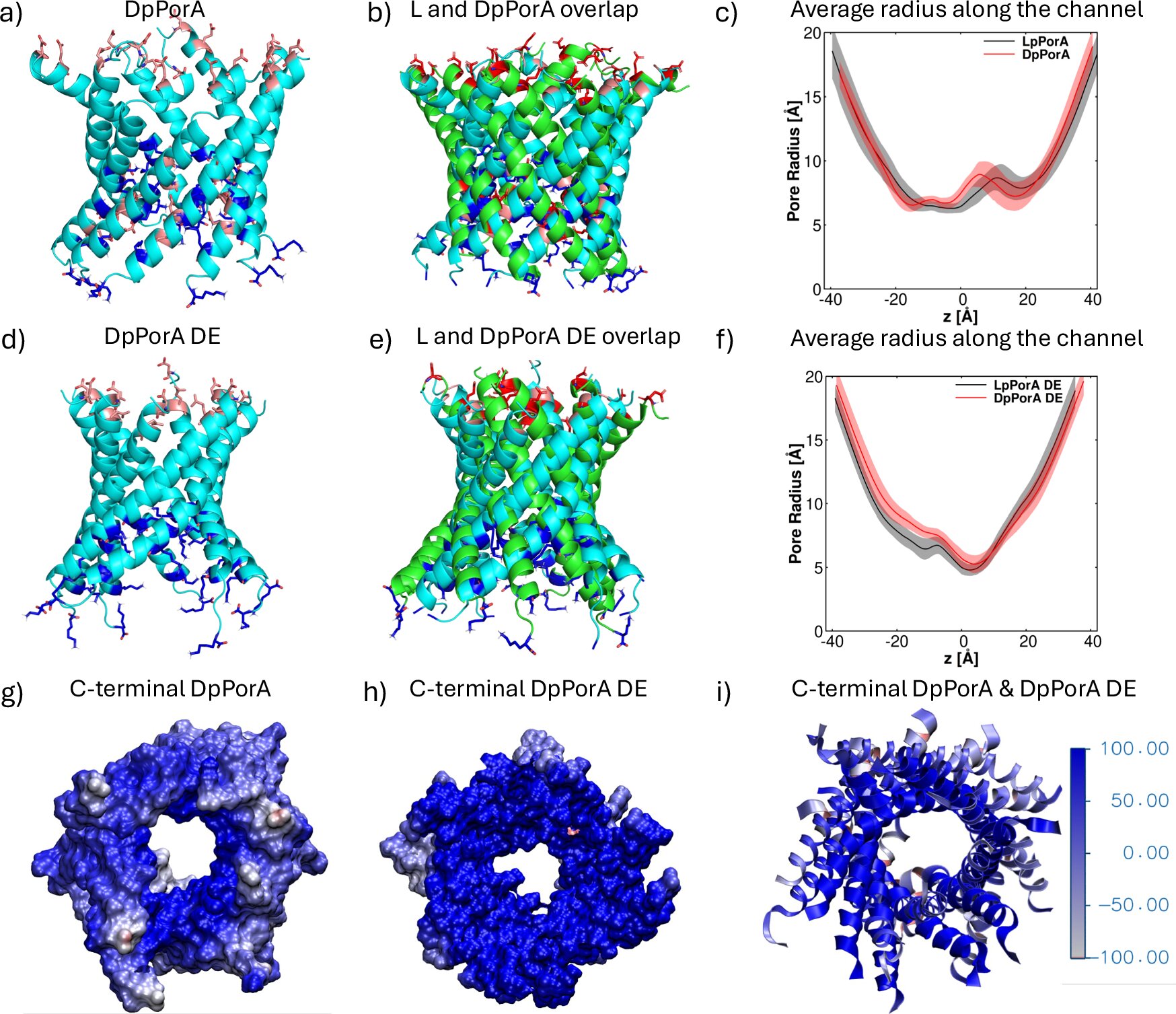
Prof. Mahendran’s team embraced this challenge and succeeded in designing a fully synthetic, stable, and functional D-peptide nanopore, aptly named DpPorA.
Building the Mirror: The Birth of DpPorA
The creation of DpPorA was not just an act of molecular craftsmanship; it was an exploration of nature’s symmetry at its finest. The team engineered this mirror-image pore with precision, ensuring that every atom corresponded perfectly to its natural L-amino acid counterpart.
But their achievement went even further. By adjusting the charge distribution — the delicate balance of positive and negative electrical forces within the pore — the researchers developed enhanced versions of DpPorA that exhibited superior conductance and selectivity under varying salt conditions.
In simpler terms, they fine-tuned how this tiny molecular doorway behaves, controlling what goes in, what stays out, and how efficiently it transports molecules — much like designing an intelligent gatekeeper for molecular traffic.
Seeing Molecules One by One
When the team tested DpPorA in the lab, they found something remarkable. These synthetic pores could detect a wide range of biomolecules at the single-molecule level, including peptides, cyclic sugars, and even certain proteins associated with neurological diseases such as Parkinson’s.
Through fluorescence imaging, scientists confirmed that DpPorA forms large, flexible channels in membranes. These channels acted as precise filters, allowing molecules of specific sizes to pass through while excluding others. This level of control — at the scale of individual molecules — is an extraordinary feat of biological engineering.
It means that one day, such pores could be used as nanoscale sensors to detect diseases at their earliest molecular signatures or to deliver drugs in highly targeted ways within the body.
The Computational Mirror: Simulating the Invisible
While the experimental work took place in India, the invisible side of this achievement unfolded thousands of kilometers away, in the virtual world of computer simulations at Constructor University in Bremen, Germany.
Here, Prof. Dr. Ulrich Kleinekathöfer, a physicist specializing in molecular simulations, and his team played a crucial role. Their high-precision computational models confirmed what could not be directly seen with the human eye — that the DpPorA pore was indeed a perfect mirror image of its natural L-form counterpart.
“Our simulations provided the molecular-level picture needed to prove that these mirror-image pores are exact counterparts of their natural analogs,” explains Prof. Kleinekathöfer. “This understanding was essential to explain the experiments and will guide the design of improved pore variants in the future.”
Dr. Kalyanashis Jana, a postdoctoral researcher and co-author of the paper, added, “The computational work gave us the confidence that we were indeed looking at a true mirror-image pore.”
The collaboration between simulation and experiment highlights one of the most beautiful aspects of modern science: how imagination, theory, and computation can merge seamlessly with real-world experiments to illuminate the unseen.
Mirror Molecules in Medicine
While the DpPorA nanopore is a scientific marvel on its own, its biomedical implications may be even more profound. When the team tested fluorescently labeled DpPorA pores in cell cultures, they found that these mirror-image structures disrupted cancer cell membranes — effectively killing cancerous cells — while leaving normal cells untouched.
This kind of selectivity is the dream of cancer researchers: a treatment that destroys malignant cells while sparing healthy ones. Though still in its early stages, this discovery hints at a future where mirror-image molecules could form the basis for targeted cancer therapies, harnessing molecular precision to fight disease without the collateral damage typical of chemotherapy.
D-proteins, with their durability and resistance to enzymatic breakdown, are already attracting attention in drug design. They could be used to create longer-lasting therapeutic molecules, vaccines, or diagnostic tools capable of surviving harsh biological environments. The DpPorA study proves that such molecular mirrors aren’t just theoretical curiosities — they can function, thrive, and perform complex biological tasks in real conditions.
A Legacy of Collaboration and Discovery
This groundbreaking work is also a story of continuity — a reflection not only of molecules but of mentorship and scientific lineage. Both Prof. Mahendran and his collaborator Dr. Harsha Bajaj earned their Ph.D.s under Prof. Dr. Mathias Winterhalter at the former Jacobs University Bremen (now Constructor University). Decades later, their collaboration with Constructor University continues, symbolizing how knowledge and mentorship transcend time and geography.
Science, like life, thrives on connection. The same spirit of collaboration that binds cells into tissues and molecules into life also binds researchers across borders. This mirror-image nanopore is, in many ways, a symbol of that connection — the reflection of shared curiosity, creativity, and perseverance.
The Dawn of a New Molecular Era
The creation of the mirror-image nanopore is more than a scientific first; it is a philosophical reminder of life’s endless capacity for reinvention. It challenges us to think beyond nature’s traditional blueprint and imagine new ways of designing molecular systems that could revolutionize medicine, biotechnology, and material science.
Mirror-image biology — the study and engineering of D-proteins and D-DNA — is still in its infancy, but its potential is vast. From developing ultrastable enzymes to crafting mirror vaccines immune to natural degradation, scientists are only beginning to explore the possibilities hidden in this molecular reflection.
The success of DpPorA marks a new horizon. It reminds us that even in the smallest details — in the handedness of a molecule — there lies a universe of untapped potential.
Reflections of the Future
As we peer deeper into the molecular mirrors of life, one truth becomes clear: biology is not static. It evolves, adapts, and learns from its own reflection. By building a mirror-image nanopore, humanity has taken a small but profound step into a parallel world — one where we can reshape the very fabric of biology to serve medicine, technology, and perhaps even the future of evolution itself.
Science has long been about understanding nature. But sometimes, to understand, we must learn to imitate — and in this case, to reflect. The mirror-image nanopore stands as both an achievement and a metaphor — proof that when we look into the molecular mirror, we not only see nature as it is, but as it could one day become.
More information: Neilah Firzan CA et al, Fabrication of cytotoxic mirror image nanopores, Nature Communications (2025). DOI: 10.1038/s41467-025-64025-6