For years, families watched their children struggle with unexplained symptoms—progressive muscle weakness, failing livers, and mounting fatigue—without answers or treatment. Doctors were left puzzled, as test after test revealed nothing conclusive. But now, a groundbreaking discovery from a team of scientists led by Duke-NUS Medical School has shed light on the cause of this mysterious illness, offering hope where before there was only uncertainty.
Published in the Journal of Clinical Investigation, the study pinpoints mutations in a gene called SPNS1 as the culprit behind this rare and previously undiagnosed condition. The revelation not only solves a medical mystery but also opens a pathway toward potential therapies.
Cracking the Code of a Hidden Disease
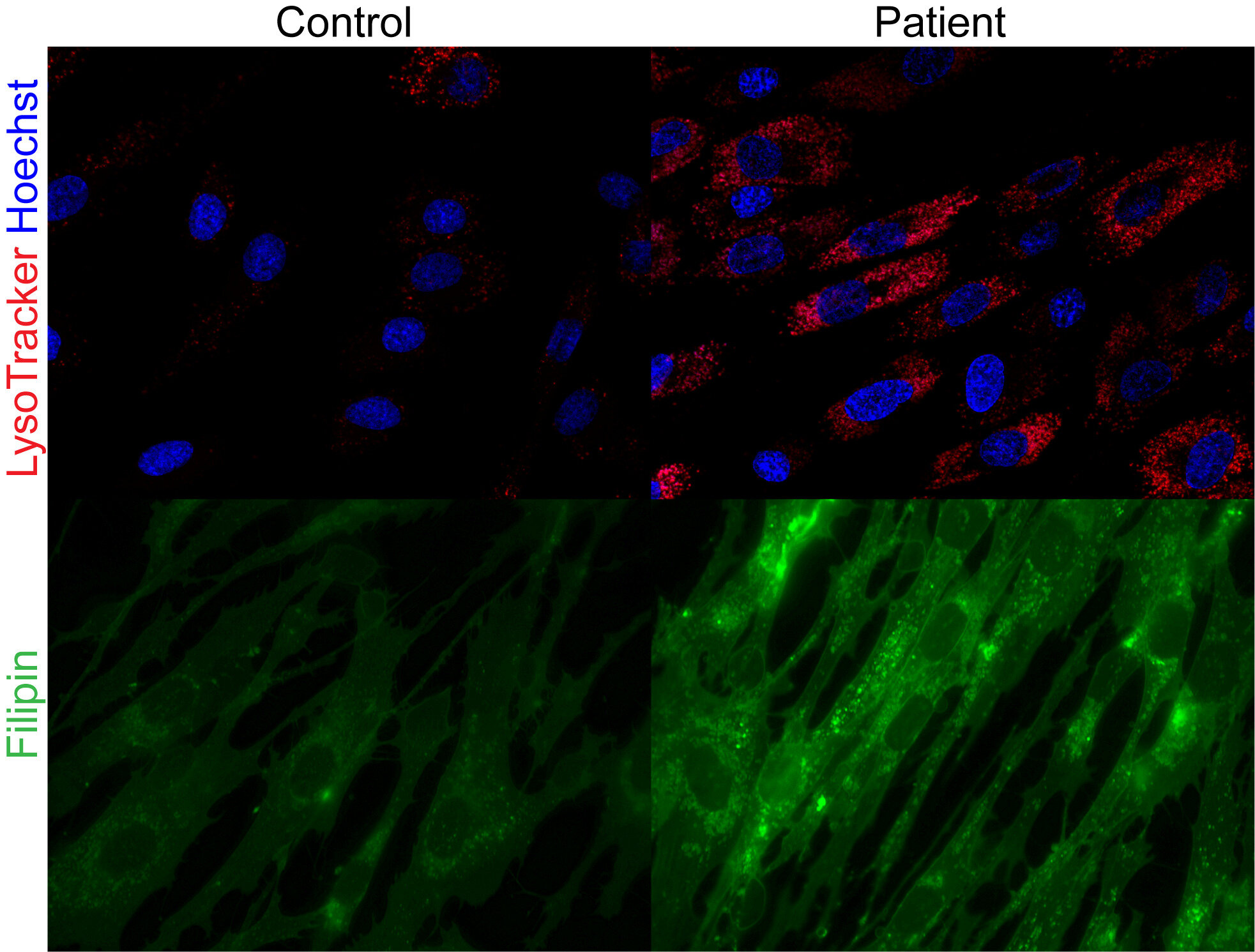
The human body has an extraordinary way of recycling its own resources. Within each cell are lysosomes, tiny structures often described as the body’s “recycling centers.” They break down fats, proteins, and other cellular materials into reusable building blocks that keep us alive and healthy.
The new research shows that in patients with SPNS1 mutations, this recycling system goes awry. Faulty SPNS1 genes disrupt the transport of phospholipids—key fat molecules used to build cell membranes—out of the lysosomes. Instead of being reused for energy or cellular repair, these fats become trapped, leading to a dangerous build-up of fat and cholesterol inside cells.
Over time, this cellular “clogging” results in damage to the muscles and liver, explaining the children’s symptoms that had baffled physicians.
Families at the Heart of Discovery
The discovery came through studying two unrelated families whose children showed similar but puzzling health problems. Genetic analysis revealed mutations in both copies of the SPNS1 gene in these children, confirming it as the root cause of their illness.
For families long searching for answers, the breakthrough was life-changing. Dalila Sabaredzovic, the mother of two affected boys, expressed both relief and optimism:
“I am so thankful that we now have a foundation to stand on and that work is progressing towards exploring paths of treatments. We feel empowered in many ways we couldn’t before and we really hope that this research will spark not only an understanding of the SPNS1 gene and the condition it’s causing, but also a way towards a cure.”
Her words underscore what this discovery means—not just a scientific achievement, but a lifeline of hope.
From Discovery to Potential Treatment
While solving the genetic mystery is a critical first step, the Duke-NUS team is determined to go further. Partnering with N = 1 Collaborative, an organization dedicated to developing personalized therapies for extremely rare diseases, the researchers are now working to translate this discovery into practical treatment.
Dr. Marlen Lauffer, a senior researcher at Leiden University Medical Center and co-author of the study, explained:
“Using what we learned from this research, we are working with the N = 1 Collaborative to create a tailored treatment for the children in our study. This work includes exploring ways to correct the faulty fat transport using new genetic therapies. Our goal is to transform scientific knowledge into therapies that improve the quality of life and give hope to other families facing similar challenges.”
By understanding the precise molecular cause of the disease, researchers can now design treatments that directly target the problem—something impossible until now.
Why SPNS1 Matters Beyond This Disease
The discovery of SPNS1’s role in fat recycling may have ripple effects far beyond this rare disorder. Professor David Silver, Deputy Director of Duke-NUS’ Cardiovascular and Metabolic Disorders Program and senior author of the study, emphasized that this is more than a single-disease breakthrough:
“SPNS1 is found in every human cell and plays a key role in recycling phospholipids. Our studies revealed that phospholipid recycling by lysosomes plays a crucial role in regulating how cells maintain normal levels of other important lipids such as fat and cholesterol. These findings open up opportunities to explore the importance of SPNS1 in other diseases such as cancer.”
In other words, solving one rare mystery may help unlock treatments for much broader health challenges, from metabolic disorders to cancer research.
The Power of Precision Medicine
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, framed the discovery as an example of the promise of precision medicine—an approach that tailors diagnosis and treatment to individual genetic profiles.
“These findings demonstrate the power of precision medicine. By linking unusual patient symptoms to specific genetic mutations, researchers uncover new disease pathways and develop targeted treatments. This approach not only provides answers to families affected by rare diseases but also opens doors for broader medical advances.”
His words capture the larger meaning of this research: science’s ability to transform the unknown into hope, and the mysterious into the solvable.
A Future Built on Hope and Collaboration
The journey from mystery symptoms to genetic discovery, and now toward tailored therapies, is a testament to what happens when families, clinicians, and scientists come together. The study’s first author, Duke-NUS MD-Ph.D. student He Menglan, described the breakthrough as “a crucial puzzle piece” in understanding a long-elusive disease.
For the affected families, it is more than a puzzle solved—it is the beginning of a new chapter. With researchers now working on targeted treatments, the possibility of real therapies feels closer than ever.
And while this rare disease affects only a handful of known patients, the insights gained ripple outward, enriching medicine’s understanding of how the human body works, how diseases arise, and how cures might be found.
In the end, this story is about more than a single gene. It is about persistence, compassion, and the extraordinary human drive to turn suffering into solutions. It is about science’s ability not just to explain, but to heal. And for the families who once had no answers, it is about a long-awaited gift: hope.
More information: Menglan He et al, SPNS1 variants cause multi-organ disease and implicate lysophospholipid transport as critical for mTOR-regulated lipid homeostasis, Journal of Clinical Investigation (2025). DOI: 10.1172/JCI193099