The integrity of our cells depends on the precise regulation of gene expression, a process that begins with the transcription of DNA into RNA. However, a groundbreaking study published in Cell reveals a hidden problem that could help explain many diseases: the failure of a gene-reading quality-control mechanism called Integrator. This defect leaves cells with abnormal RNA strands that increase cellular stress and could contribute to a variety of conditions, including cancer, neurodegeneration, and developmental abnormalities. The research, led by Karen Adelman and her team at Harvard Medical School, offers a fresh perspective on the role of incomplete RNA in disease and suggests new potential treatments.
Integrator and Its Vital Role in Gene Expression
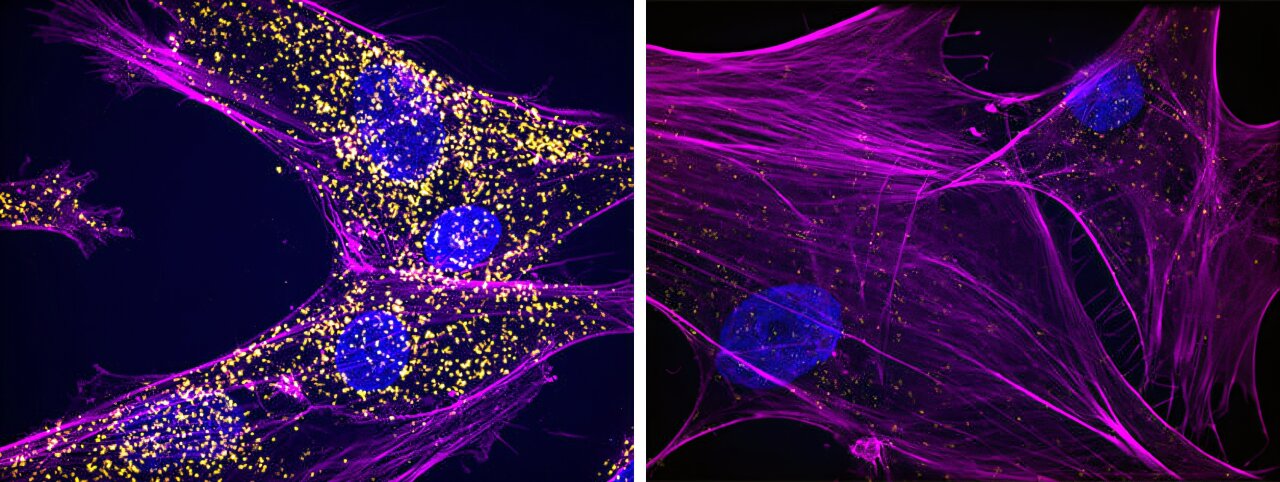
At the heart of this research is Integrator, a protein complex responsible for ensuring the complete and proper transcription of genes. In simple terms, Integrator ensures that when a gene is “read” by the cellular machinery, the process finishes correctly, avoiding incomplete or faulty RNA strands. Without proper completion, these incomplete RNA molecules could lead to disastrous consequences for the cell.
To illustrate the importance of Integrator, imagine a race where the runners are the RNA molecules, and the finish line is the complete, mature RNA that will be used to create proteins. Integrator functions as a race official who ensures that only qualified runners (or RNA molecules) complete the marathon. But when Integrator malfunctions, it’s like allowing an unqualified participant—someone unable to finish the race—to enter. This results in incomplete RNAs, which contain sequences called introns that should not be present in the final RNA product.
It was long assumed that such faulty RNAs would simply be destroyed by the cell’s quality control mechanisms before they could do any harm. However, Adelman and her team discovered a far more concerning reality.
Incomplete RNAs: A Hidden Threat to Cell Health
Despite being incomplete, some of these faulty RNA strands manage to escape the nucleus, the part of the cell where they are normally processed. Once in the cytoplasm, these incomplete RNA molecules can fold back on themselves and create double-stranded RNA. Normally, double-stranded RNA is a sign of viral infection, as the cell recognizes this abnormal structure as a potential pathogen.
This discovery revealed a hidden layer of cellular stress. When the body detects double-stranded RNA, it triggers the integrated stress response (ISR), a defense mechanism that helps protect the cell but can also have harmful side effects when activated chronically. This stress pathway has been implicated in a wide range of diseases, including neurodegeneration, cancer, and aging. Importantly, this finding could explain some of the puzzling symptoms seen in patients with mutations in the Integrator complex—symptoms that could not previously be directly tied to gene activity.
“Normally, you can look at the altered gene activity in patients with a mutation and understand how you get from there to the patient’s symptoms. But with Integrator, the gene activity doesn’t explain the symptoms,” said Karen Adelman, senior author of the study. Instead, it’s the accumulation of incomplete RNA that causes the cellular stress, contributing to disease.
Investigating the Stress Pathway and Its Effects
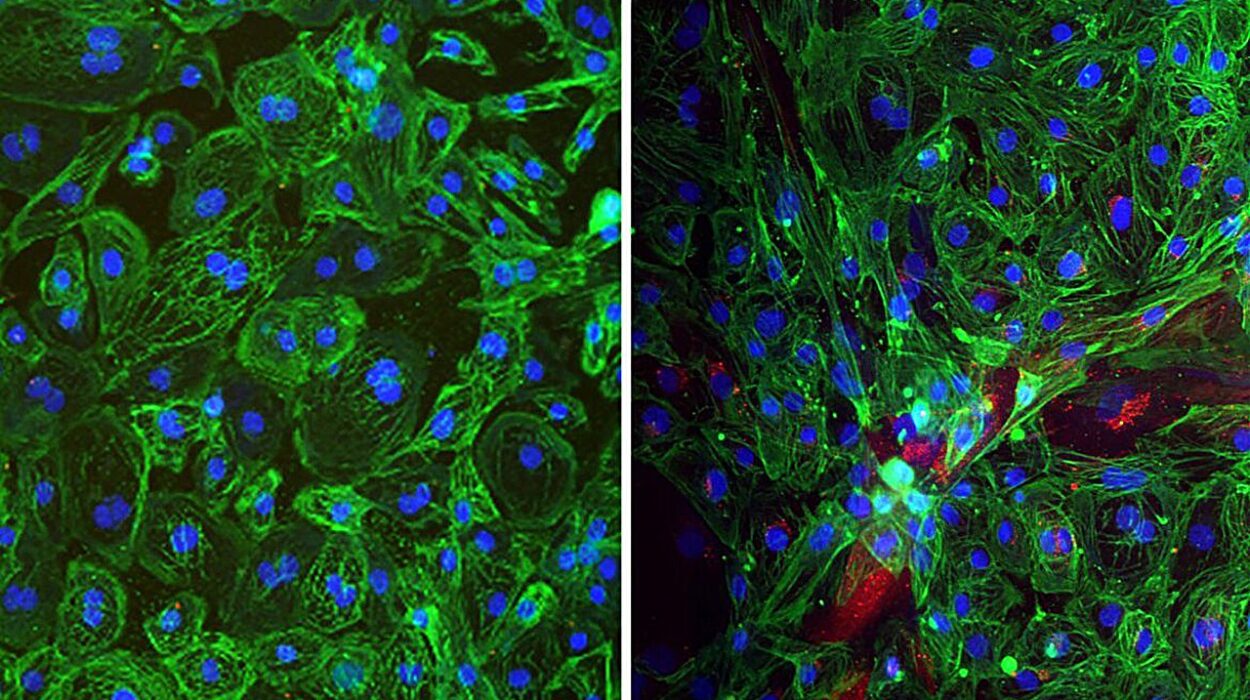
The study’s authors used cell cultures and tissue samples from patients to delve deeper into the consequences of Integrator mutations. They found that the faulty RNA in these patients not only activated the ISR but also resulted in elevated stress levels within cells, which could eventually lead to cell damage or death.
These findings were particularly striking because they highlight the importance of previously overlooked factors in gene transcription. While research has long focused on measuring the amount of mature, full-length RNA produced by genes, the study suggests that the immature and incomplete RNA molecules—the ones often discarded as unimportant—may be the very culprits behind cell dysfunction. This new perspective shifts the focus to include these discarded RNA products in understanding diseases.
Potential Treatments: Targeting PKR to Relieve Stress
Despite the troubling implications of the study, there is a silver lining. The research team identified a molecule called PKR (protein kinase R), which is responsible for triggering the stress response when it detects double-stranded RNA. When the researchers inhibited PKR in their experiments, they found that the stress response was significantly reduced, alleviating some of the damage caused by the faulty RNA.
This discovery opens the door for potential treatments targeting PKR to mitigate the effects of Integrator mutations and similar gene expression failures. Some PKR inhibitors are already being developed, and studies have even suggested that existing drugs, such as the diabetes medication metformin, may suppress PKR activity. This could offer a fast track to treatments for diseases associated with Integrator and other similar transcriptional regulators.
Double-Stranded RNA: A New Diagnostic Opportunity
A key challenge in diagnosing diseases caused by Integrator mutations is the difficulty of detecting incomplete RNA. Most scientific tests focus on mature, full-length RNA, so the immature, faulty RNA responsible for causing stress has largely remained under the radar. However, Adelman’s team developed a novel method to specifically study these incomplete RNAs, opening up new diagnostic possibilities.
“We’re hearing from clinicians who haven’t been satisfied with the understanding of how mutations in Integrator or other transcription regulators cause their patients’ disease,” said Adelman. Many of these clinicians are now seeking advice from her lab on how to test patient samples for these abnormal RNA strands. This indicates that the findings of the study could lead to a new avenue for diagnosing disorders caused by malfunctioning transcription machinery.
Moreover, the discovery of double-stranded RNA as a cause of disease could help clarify why some mutations in gene transcription regulators seem to disproportionately affect neurons. Neuronal genes are some of the longest in the human genome, and the defective machinery responsible for reading these genes is least likely to finish the “marathon” of transcription, leading to incomplete RNA and increased stress in these critical cells. This could explain the neurological symptoms seen in patients with Integrator mutations.
The Broader Implications of Integrator Mutations
While this study provides important insights into the mechanisms underlying Integrator-related diseases, the researchers also emphasize that it is just one piece of the puzzle. Double-stranded RNA will not explain every disorder linked to malfunctions in RNA production, but it adds a critical layer to our understanding of gene expression and cellular stress.
In particular, the study highlights the need to expand the scope of research and clinical diagnostics to include the role of incomplete RNA. This is especially important in cases where a patient’s symptoms do not align neatly with their genetic profile. As researchers and clinicians begin to explore this new avenue, it could lead to better-targeted therapies and more effective treatments for diseases that currently have no cure.
In the end, the study opens new doors for understanding how failures in RNA quality control can contribute to a wide array of diseases, and it suggests that interventions targeting the cellular stress response could hold promise for treating disorders that have long stumped medical professionals. With this fresh insight into the role of Integrator in gene expression, a more complete picture of the underlying causes of many diseases is now beginning to emerge.
Reference: Apoorva Baluapuri et al, Integrator loss leads to dsRNA formation that triggers the integrated stress response, Cell (2025). DOI: 10.1016/j.cell.2025.03.025