Chemistry, at its heart, is a story of relationships—between atoms, electrons, and energy. Among the most powerful ideas that govern these relationships is a seemingly simple rule that has profound implications for everything from table salt to the DNA in your cells: the Octet Rule.
This rule, centering around the number eight, underpins much of what makes chemistry both predictable and beautifully complex. It explains why atoms form bonds, how molecules take shape, and why certain elements behave the way they do. But as with all rules in science, the closer you look, the more fascinating exceptions and subtleties you uncover.
In this article, we will journey through the foundations, applications, limitations, and deeper implications of the Octet Rule. We’ll delve into the quantum world, explore electron configurations, learn how the rule shapes the periodic table, and even look at how it breaks down in exotic compounds. Whether you’re a curious student, a lifelong learner, or someone enchanted by the unseen world of atoms, this exploration will illuminate one of chemistry’s most powerful organizing principles.
What Is the Octet Rule?
At its core, the Octet Rule states:
Atoms tend to gain, lose, or share electrons in order to have eight electrons in their outer (valence) shell, achieving the electron configuration of a noble gas.
This tendency drives atoms to form chemical bonds. It’s why sodium (Na) and chlorine (Cl) come together to make table salt (NaCl). Sodium loses one electron (ending up with 8 in the previous shell), and chlorine gains one (reaching 8 in its outer shell), forming a stable compound.
The rule is most relevant to the main-group elements (s- and p-block elements), especially those in periods 2 and 3 of the periodic table. Noble gases—such as neon (Ne) and argon (Ar)—already have full outer shells, and so are chemically inert under most conditions. They represent the “ideal” that other atoms strive toward.
But what makes eight so special?
To answer that, we must dive into the realm of electron configurations and atomic structure.
Atomic Structure and Electron Shells: Building to Eight
Atoms consist of a nucleus (protons and neutrons) surrounded by electrons. These electrons occupy regions called energy levels or shells, which are further divided into subshells and orbitals.
The first shell (n=1) holds up to 2 electrons.
The second shell (n=2) holds up to 8 electrons.
The third shell (n=3) begins to hold more—but the first 8 are crucial for chemical behavior.
Electrons fill orbitals in a specific sequence:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p…
Each orbital can hold 2 electrons, and the s and p orbitals in a given energy level account for the maximum of 8 valence electrons:
- 2 electrons in the s orbital
- 6 electrons in the three p orbitals
This is the foundation of the Octet Rule. Atoms are most stable when their outermost s and p orbitals are completely filled—totaling 8 electrons.
Why Atoms Obey the Octet Rule
Atoms “prefer” to have full outer shells because:
- Lower energy equals greater stability – Atoms tend to move toward arrangements of lowest potential energy.
- Full shells resemble noble gas configurations – Noble gases have full valence shells and do not readily react. Atoms imitate this stability.
- Coulombic attractions drive charge balance – Atoms form ions or share electrons to balance attractions and repulsions between charged particles.
In essence, chemical bonds form because atoms seek stable, low-energy configurations. The Octet Rule is a shortcut for predicting how atoms behave in this quest for stability.
Types of Bonding Explained by the Octet Rule
Let’s explore how the Octet Rule plays out in real-world chemical bonding:
Ionic Bonding
In ionic bonding, atoms transfer electrons to achieve octets.
Example: Sodium Chloride (NaCl)
- Sodium (Na): 1 valence electron → loses it to achieve an octet (now like Ne)
- Chlorine (Cl): 7 valence electrons → gains 1 to reach 8 (now like Ar)
- Result: Na⁺ and Cl⁻ ions form a strong electrostatic bond
Ionic compounds often form between metals (which lose electrons) and nonmetals (which gain electrons). The Octet Rule predicts the charges these ions will adopt.
Covalent Bonding
In covalent bonding, atoms share electrons to complete their octets.
Example: Water (H₂O)
- Oxygen has 6 valence electrons → needs 2 more
- Each hydrogen provides 1 → sharing creates two O–H bonds
- Oxygen ends up with 8 valence electrons, each hydrogen has 2 (duet rule)
Covalent bonds are most common between nonmetals and are governed by the desire of all involved atoms to reach stable electron configurations.
Coordinate Covalent Bonds
These are special covalent bonds in which one atom donates both electrons for the shared pair. Once formed, the bond is indistinguishable from any other covalent bond.
Example: Ammonium ion (NH₄⁺)
- Ammonia (NH₃) has a lone pair on nitrogen
- A proton (H⁺) accepts this pair, forming NH₄⁺
- All atoms now satisfy the Octet Rule
The Octet Rule guides the logic behind which atoms can accept or donate electron pairs.
The Octet Rule in Action: Molecular Examples
Let’s break down some more examples to see how the Octet Rule applies:
Methane (CH₄)
- Carbon has 4 valence electrons → forms 4 covalent bonds with hydrogen
- Each H shares 1 electron → all atoms reach full outer shells (C has 8, each H has 2)
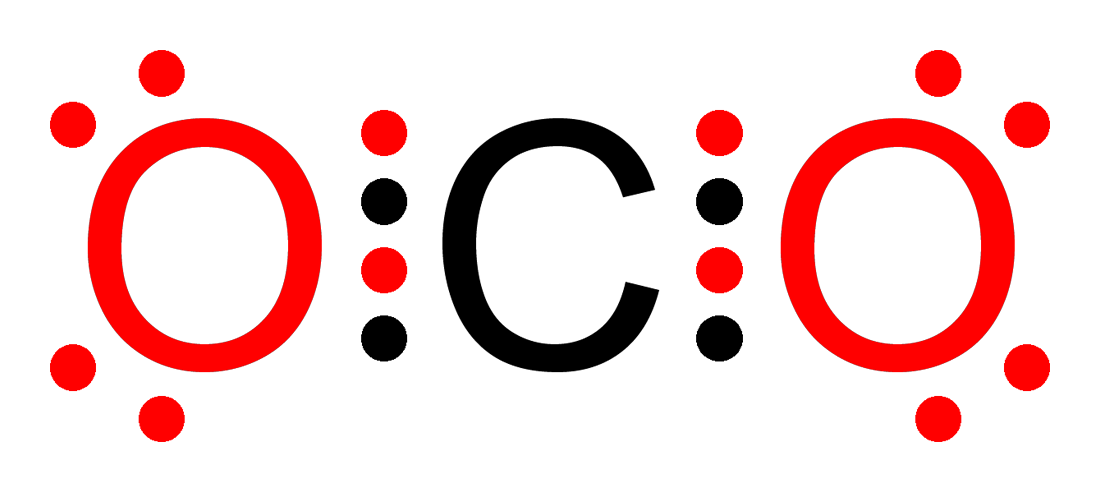
Carbon Dioxide (CO₂)
- Carbon forms two double bonds with two oxygen atoms
- Each atom ends up with 8 valence electrons
- The molecule is linear and stable
Nitrogen (N₂)
- Nitrogen atoms triple bond with each other (N≡N)
- Each N shares 3 electrons → both reach octets
- Very strong, nonreactive bond
These molecules are foundational to life and industrial chemistry, and the Octet Rule helps explain why they’re so stable.
Exceptions to the Octet Rule
Chemistry, like life, is full of exceptions. While the Octet Rule holds for many atoms, it’s not universal.
1. Incomplete Octets
Some atoms are stable with fewer than 8 electrons, especially small ones:
- Hydrogen: 2 electrons (duet rule)
- Helium: already full with 2 electrons
- Beryllium (Be): often stable with 4 electrons
- Boron (B): often stable with 6 electrons
Example: BF₃ (boron trifluoride)
- Boron only has 6 valence electrons
- Molecule is stable and symmetrical
These atoms are electron-deficient and often behave as Lewis acids, accepting lone pairs from other atoms.
2. Expanded Octets
Atoms in Period 3 or below can hold more than 8 electrons in their valence shells due to accessible d orbitals.
Examples:
- Phosphorus pentachloride (PCl₅) – 10 valence electrons on P
- Sulfur hexafluoride (SF₆) – 12 valence electrons on S
- Xenon tetrafluoride (XeF₄) – 12 valence electrons on Xe
These are valid Lewis structures, and these molecules are stable despite “breaking” the Octet Rule.
3. Odd-Electron Molecules
Some molecules have an odd number of electrons, resulting in radicals.
Examples:
- Nitric oxide (NO) – 11 total valence electrons
- Nitrogen dioxide (NO₂) – 17 valence electrons
Such molecules are highly reactive, often short-lived, and play major roles in atmospheric chemistry and biology.
Visualizing Octets: Lewis Structures and the Octet Rule
One of the most useful tools in chemistry for applying the Octet Rule is the Lewis structure, a simple diagram showing bonds and lone pairs.
Steps to draw a Lewis structure:
- Count total valence electrons.
- Determine the central atom.
- Connect atoms with single bonds.
- Distribute remaining electrons to complete octets (or duets for hydrogen).
- Use double/triple bonds if needed to satisfy the octet.
Let’s try carbon dioxide (CO₂):
- Total valence electrons: 4 (C) + 6×2 (O) = 16
- Place C in center, connect to two O atoms
- Add lone pairs to each O
- Adjust with double bonds to give carbon 8 electrons
The resulting structure satisfies the Octet Rule for all atoms.
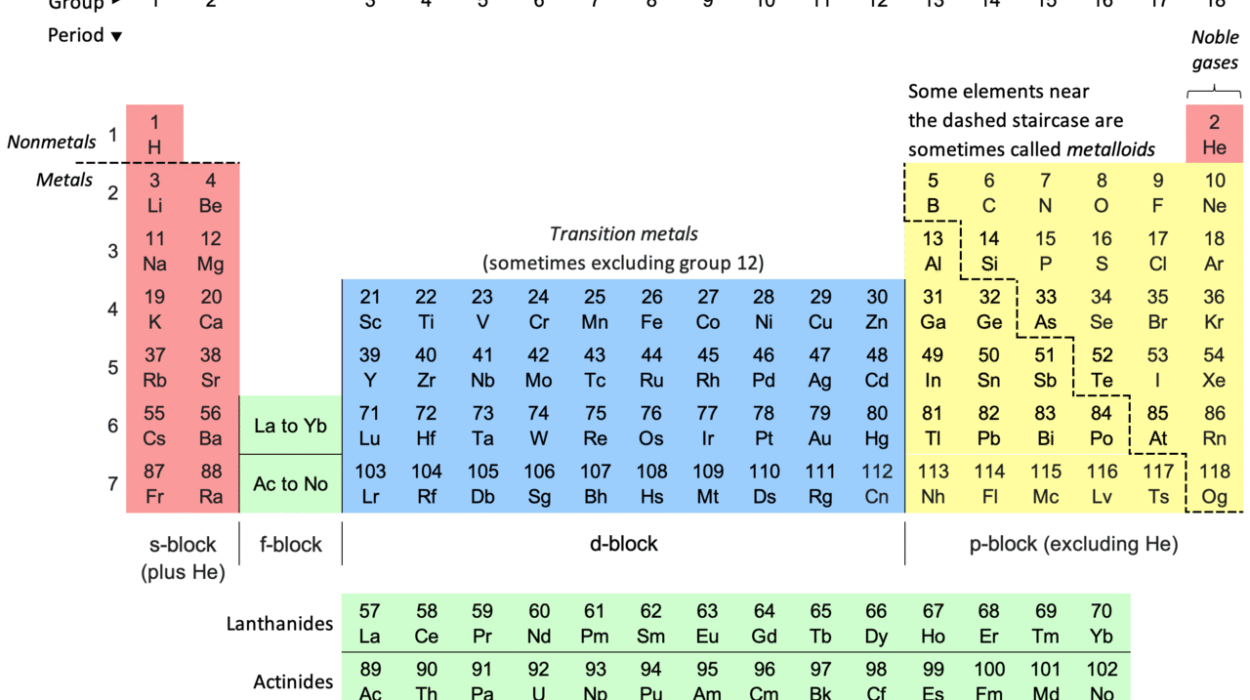
The Octet Rule and the Periodic Table
The periodic table is not random—it’s a systematic arrangement of elements based on electron configuration, and the Octet Rule helps explain its shape.
- Group 1 (alkali metals): 1 valence electron → lose it to form +1 ions
- Group 2 (alkaline earth metals): 2 valence electrons → lose 2 to form +2 ions
- Group 17 (halogens): 7 valence electrons → gain 1 to form –1 ions
- Group 18 (noble gases): full octets → unreactive
You can predict reactivity, bonding behavior, and oxidation states just by knowing the group number—thanks to the Octet Rule.
Real-Life Applications of the Octet Rule
This isn’t just theory. The Octet Rule helps explain and predict countless phenomena in the world around you.
In Biology
- DNA base pairing relies on hydrogen bonds and molecular geometry guided by electron configuration.
- Proteins fold based on interactions influenced by octet-based bonding patterns.
- Cell membranes are stabilized by amphipathic molecules with polar and nonpolar regions shaped by electron sharing.
In Medicine
- Drug design depends on understanding molecular shape and electron distribution.
- Metal ions in enzymes (like Fe²⁺ in hemoglobin) follow bonding rules rooted in octet concepts.
In Industry
- Creating polymers like plastics involves covalent bonding and Octet Rule-based monomer design.
- Battery chemistry (e.g., lithium-ion) is driven by electron transfer to achieve noble gas configurations.
Beyond the Octet: Hypervalency and Advanced Bonding Theories
As you dive deeper into chemistry, you’ll find that the Octet Rule is a starting point—not an end.
Hypervalent molecules, resonance structures, molecular orbital theory, and quantum chemistry provide a more complete picture. Concepts like delocalized electrons, hybridization, and sigma/pi bonds go far beyond the basic octet idea.
Still, the Octet Rule remains a powerful heuristic—a gateway to understanding molecular structure, stability, and reactivity.
Conclusion: The Power and Poetry of Eight
The Octet Rule is more than just a number or a rule—it’s a window into the soul of the atom. It explains the silent dance of electrons that holds matter together, gives shape to the molecules of life, and allows us to predict and manipulate the material world.
Like all great scientific principles, its beauty lies in its simplicity and depth. With just the number eight, it ties together the structure of the periodic table, the formation of chemical bonds, and the behavior of matter across the universe.
Whether you’re mixing table salt, studying atmospheric gases, or dreaming of alien biochemistry, the Octet Rule is there—quietly guiding the architecture of atoms and the elegance of everything that follows.