For a few dozen people around the world, living with a rare immune disorder has meant facing a heightened risk of certain bacterial infections. But this biological downside comes with a startling upside: the apparent ability to fend off every virus that comes their way.
Fifteen years ago, Columbia University immunologist Dusan Bogunovic first encountered these individuals and began unraveling their mysterious resistance. All of them share a mutation that disrupts the immune regulator ISG15. At first, the mutation seemed only to weaken defenses against some bacteria. But as more patients surfaced, a surprising pattern emerged—despite clear evidence their immune systems had fought flu, measles, mumps, and even chickenpox, none of them recalled ever falling ill.
“The type of inflammation they had was antiviral,” Bogunovic explains. “That’s when it dawned on me that these individuals could be hiding something. Their bodies were essentially living in a constant state of mild antiviral defense.”
Turning biology into therapy
Bogunovic’s latest study, published August 13 in Science Translational Medicine, translates this natural superpower into a potential therapy. His team has designed an experimental treatment that temporarily reproduces the same protective state in animals.
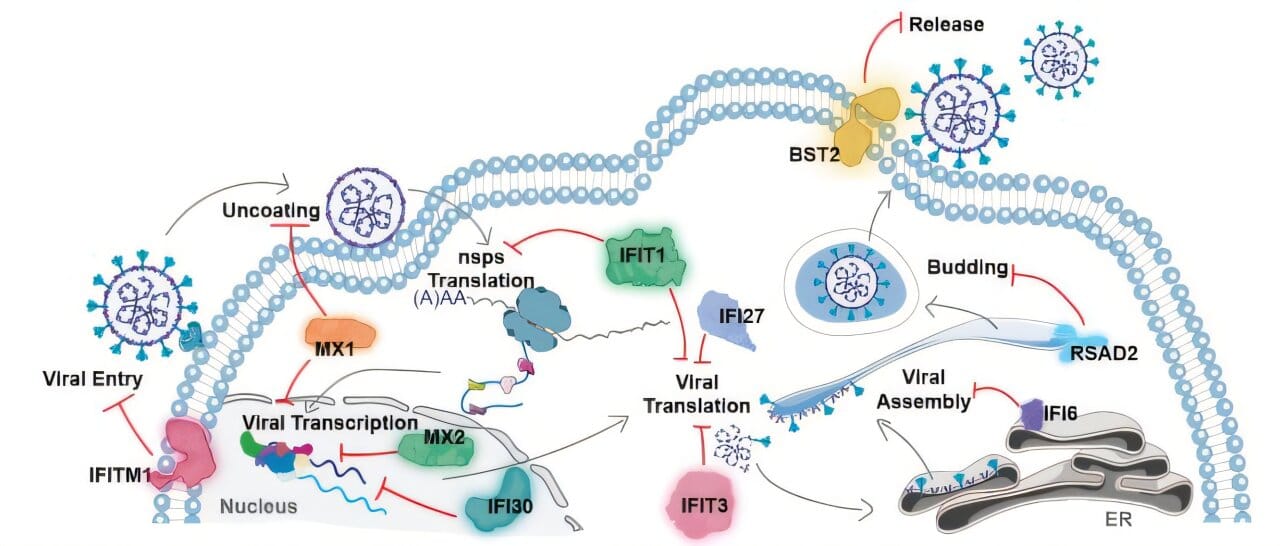
In tests with mice and hamsters, a simple nasal drip carrying the therapy blocked viral replication of influenza and SARS-CoV-2, the virus behind COVID-19. The treated animals not only avoided severe illness but also showed broad-spectrum resistance. In cell culture, no virus tested so far—including coronaviruses and flu strains—has managed to break through.
“Our goal is not to permanently mimic ISG15 deficiency,” Bogunovic says, “but to copy its most protective features for a short window of time.”
The therapy works by instructing cells to briefly manufacture ten antiviral proteins, chosen from more than 60 produced in ISG15-deficient individuals. Delivered via a lipid nanoparticle—similar to the technology behind COVID mRNA vaccines—the therapy sparks just enough inflammation to block viral disease, but not so much as to cause harmful side effects.
A universal antiviral in the making
The implications could be transformative. Unlike vaccines, which are tailored to specific pathogens, this approach could provide immediate defense against almost any virus—even unknown ones. That could make it a critical tool in the early days of the next pandemic, offering frontline protection for health workers, vulnerable communities, and families of infected individuals.
“What excites us most,” Bogunovic says, “is that the technology could work even if we don’t know the identity of the virus. It provides a safety net.”
Importantly, the treatment would not prevent the body from developing its own long-term immunity after exposure, meaning patients could still build natural protection.
Hurdles before human use
Challenges remain before the therapy can be tested in people. Delivering nucleic acids like mRNA efficiently into the lungs is notoriously difficult. In current experiments, the antiviral proteins appeared in lung cells, but not at the levels scientists believe are necessary for reliable human protection. Researchers must also determine how long the protective window lasts—right now, it appears to be only three to four days.
“Once the therapy reaches our cells, it works,” Bogunovic notes. “But delivery is the biggest challenge in the field today.”
A discovery born of curiosity
What began as an investigation into an obscure genetic disorder has blossomed into a blueprint for universal antiviral defense. For Bogunovic, the story underscores the importance of open-ended science.
“Our findings reinforce the power of research driven by curiosity without preconceived notions,” he says. “We weren’t looking for an antiviral when we began studying our rare patients. But those patients have inspired us to imagine a future where everyone might share their superpower.”
If successful, that future could transform the way humanity prepares for viral threats—turning a rare medical vulnerability into one of the most powerful shields against pandemics.
More information: Yemsratch Akalu et al, An mRNA-based broad-spectrum antiviral inspired by ISG15 deficiency protects against viral infections in vitro and in vivo, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adx5758