Atoms and molecules are the building blocks of everything we see, touch, and interact with—from the air we breathe to the cells in our bodies to the materials in the device you’re using to read this. But despite their importance, atoms are invisible to the naked eye, and even the best microscopes can’t reveal the buzzing energy that governs how atoms bond and behave.
So how do we, as mere humans, make sense of something we can’t see?
Enter Lewis Dot Structures, one of the most elegant and powerful tools in chemistry. These diagrams may look like mere scribbles of letters and dots, but they represent the inner logic of molecules. They help us peer into the electron cloud and decode the behavior of matter at its most fundamental level.
This article will take you on a deep, engaging journey through the world of Lewis Dot Structures: where they come from, how they work, why they matter, and how they unlock the secrets of molecules in a way few other tools can. Along the way, we’ll explore not just the method, but also the stories, theories, and insights that have shaped this cornerstone of chemistry.
Let’s begin.
The Origins: Who Was Lewis and What Did He See?
The “Lewis” in Lewis Dot Structures refers to Gilbert N. Lewis, an American physical chemist who, in the early 20th century, revolutionized how chemists thought about atoms and bonding.
Born in 1875, Lewis was a trailblazer. He didn’t just want to know what atoms were—he wanted to understand how and why they came together to form the structures of matter. At the time, the quantum model of the atom was still in its infancy, and much of chemical bonding was still a mystery.
In 1916, Lewis proposed a new, groundbreaking idea: atoms form bonds by sharing pairs of electrons. This simple yet profound insight laid the foundation for what we now call valence bond theory.
To help visualize this concept, he developed a notation—a way to sketch out atoms and their valence electrons using dots around the chemical symbols. With just dots and lines, chemists could suddenly visualize complex ideas about atomic interaction, bonding, and molecular geometry.
It was, in a word, revolutionary.
The Language of Dots: What Is a Lewis Structure?
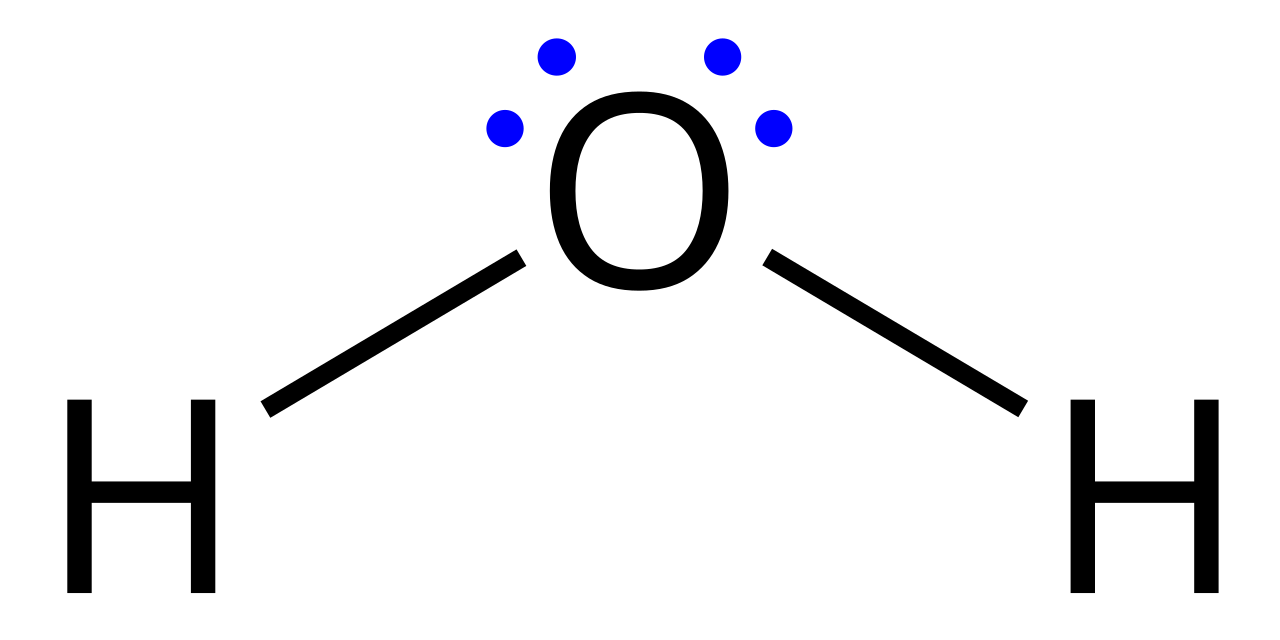
A Lewis Dot Structure (also called a Lewis structure or electron dot structure) is a diagram that shows how valence electrons (the outermost electrons of an atom) are arranged among atoms in a molecule.
Each element’s symbol represents the nucleus and inner electrons of the atom. Around this symbol, dots are placed to indicate valence electrons. When atoms bond, dots are paired—either as shared pairs (bonds) or lone pairs (non-bonding electrons).
These dots and lines are more than decoration. They convey:
- How many bonds an atom forms
- What kind of bonds (single, double, triple)
- Where the electrons are in a molecule
- The molecular shape, charge distribution, and potential reactivity
It’s a powerful, minimalist form of communication—a kind of molecular shorthand.
Valence Electrons: The Key to Bonding
To understand Lewis structures, we must understand valence electrons. These are the electrons in the outermost shell of an atom and are responsible for chemical bonding.
The periodic table, far from being just a list of elements, is a map of electron configurations. Group 1 elements have 1 valence electron, group 2 has 2, and so on, up to group 18 (the noble gases) with 8 valence electrons (except helium, which has 2).
Atoms “want” to achieve a stable configuration—typically the octet rule, which states that atoms tend to gain, lose, or share electrons to have 8 electrons in their valence shell. This is why sodium (Na) donates 1 electron to chlorine (Cl): so both reach a stable octet.
Lewis Dot Structures visualize this electron exchange or sharing.
Drawing Lewis Structures: Step-by-Step Logic
Though they can represent complex ideas, Lewis structures follow a surprisingly logical set of steps. Let’s walk through the process using the example of CO₂ (carbon dioxide).
- Count the total valence electrons
Carbon has 4, and each oxygen has 6 → 4 + 6×2 = 16 valence electrons total. - Determine the central atom
Usually the least electronegative atom. Here, carbon is central. - Connect atoms with single bonds
Each single bond = 2 electrons. Carbon forms two single bonds with oxygen → 4 electrons used. - Distribute remaining electrons as lone pairs
Place remaining 12 electrons (16 – 4) around outer atoms first. Fill octets. - Check octets and form double/triple bonds if needed
Oxygen has 8, but carbon only has 4 → form double bonds with both oxygens. Now carbon has 8. - Verify total electron count
2 double bonds = 4 electrons each = 8; 4 lone pairs (2 per O) = 8 → 8 + 8 = 16. - Finalize structure
O=C=O with two lone pairs on each O. Done!
This systematic process can be used for countless molecules, from simple H₂O to complex organic structures.
Single, Double, and Triple Bonds: What They Mean
Lewis structures don’t just show how atoms are linked—they show how strongly they are linked.
- A single bond (two electrons) is the simplest and most flexible.
- A double bond (four electrons) is stronger, shorter, and less flexible.
- A triple bond (six electrons) is very strong and very short.
For example, N≡N (nitrogen gas) is a stable triple bond, making it largely inert. The double bonds in O₂ (oxygen) allow it to be reactive enough to support combustion and respiration.
These bonds explain a molecule’s stability, reactivity, and even color or smell.
Resonance: When One Structure Isn’t Enough
Some molecules can’t be accurately represented by a single Lewis structure. Enter resonance—the idea that a molecule can be depicted as a blend of two or more valid structures.
A classic example is benzene (C₆H₆). Its six carbon atoms form a ring with alternating single and double bonds—but in reality, all bonds are identical, with electron density spread evenly.
Lewis structures represent this with resonance forms connected by a double-headed arrow. These forms don’t flip back and forth; instead, the molecule is a hybrid of them all.
Resonance structures help explain real-world properties like:
- Delocalized electrons in aromatic compounds
- Stability in polyatomic ions like NO₃⁻
- Unique reactivity of carboxylic acids, esters, and amides
Formal Charge: Keeping Track of Electron Economics
Sometimes, multiple Lewis structures seem equally valid. How do we decide which one is “best”?
One tool is formal charge, which is a hypothetical charge assigned to atoms in a molecule, assuming electrons are shared equally.
Formal Charge = (Valence electrons) – (Non-bonding electrons) – ½(Bonding electrons)
The best structure usually:
- Has formal charges close to zero
- Places negative charges on more electronegative atoms
- Avoids placing like charges next to each other
Consider ozone (O₃): two structures have identical atoms but different formal charges. Calculating formal charges helps us select the most accurate resonance hybrid.
Lewis Structures in Ions and Polyatomic Species
Lewis structures aren’t just for neutral molecules—they’re crucial for ions.
Take sulfate (SO₄²⁻): sulfur is bonded to four oxygen atoms and carries an overall charge of -2. Drawing this structure requires accounting for extra electrons, and the resonance hybrid reveals why all four S–O bonds are equivalent in length.
Polyatomic ions like NH₄⁺, NO₃⁻, and HCO₃⁻ are often components of acids, bases, and salts. Lewis structures help predict:
- How these ions interact
- Their geometries
- Where charges are concentrated
- Whether they’re reactive or stable
VSEPR and Shape: Beyond the Dots
Lewis structures are two-dimensional, but molecules exist in three.
To understand the 3D shape of molecules, chemists use Valence Shell Electron Pair Repulsion (VSEPR) theory, which builds directly on Lewis structures.
VSEPR states that electron pairs (bonding or lone) repel each other and arrange themselves as far apart as possible. Based on the number of electron regions around a central atom, we get:
- 2 regions → linear (CO₂)
- 3 regions → trigonal planar (BF₃)
- 4 regions → tetrahedral (CH₄)
- 5 regions → trigonal bipyramidal (PCl₅)
- 6 regions → octahedral (SF₆)
So while Lewis structures give us the foundation, VSEPR helps visualize the real-world molecule—its shape, angle, and symmetry.
Electronegativity and Polarity: Who’s Hogging the Electrons?
Not all bonds are equal. Some atoms are greedier than others—this tendency is called electronegativity.
In a Lewis structure, bonds between atoms with differing electronegativities result in polar bonds, which means electrons are pulled closer to one atom. This asymmetry can lead to molecular polarity, which affects everything from solubility to boiling point to how molecules interact in biological systems.
For example:
- Water (H₂O) has polar O–H bonds and a bent shape → it’s polar.
- Carbon dioxide (CO₂) has polar C=O bonds, but a linear shape → it’s nonpolar.
Lewis structures help us see where charges accumulate, which molecules will attract or repel, and how they’ll behave in chemical reactions.
Lewis Structures in Organic Chemistry
Organic chemistry—the chemistry of carbon—is practically built on Lewis structures.
In fact, the backbone of organic molecules—chains of carbon and hydrogen—is best understood through this model. Functional groups like alcohols (-OH), amines (-NH₂), carboxylic acids (-COOH), and ketones (=O) are easily visualized using Lewis dots.
Reaction mechanisms, a central concept in organic chemistry, often depend on the movement of electrons, shown with curved arrows derived from Lewis structures.
Understanding these diagrams is essential for:
- Predicting reactivity
- Designing drugs
- Understanding biological molecules like DNA and proteins
- Interpreting spectroscopy data
Biology and Biochemistry: Life Through the Lens of Lewis
In biological systems, molecular structure is destiny.
Enzymes fold and function because of specific bond angles and charges. DNA replicates because of hydrogen bonding and base pairing. Cell membranes form due to polarity and hydrophobic interactions.
All these phenomena can be traced back to basic Lewis structures:
- Hydrogen bonds (O–H···O) are visualized via lone pairs
- Base pairing (A–T, G–C) involves specific hydrogen donors and acceptors
- Proteins fold based on charged side chains and polar/nonpolar interactions
Without Lewis structures, these fundamental insights would be far more obscure.
Modern Chemistry and Quantum Backing
While Lewis structures are a simplified, classical model, they remain useful even in modern quantum chemistry. In fact, quantum calculations often begin with a Lewis guess for electron configuration.
Advanced models like:
- Molecular Orbital Theory
- Density Functional Theory
- Hartree–Fock approximations
…build on the principles captured in Lewis structures, refining them with computational precision. But even these advanced models still represent bonding in terms that trace back to shared pairs of electrons and octets.
In other words, Lewis structures aren’t obsolete—they’re foundational.
The Pedagogical Power of Dots
In the classroom, Lewis structures are a rite of passage. Students learn to think about atoms not as isolated spheres, but as parts of systems governed by rules and relationships.
The simplicity of drawing them on paper teaches:
- The concept of conservation (of charge, electrons)
- The balance between stability and reactivity
- The geometry of molecules
- The power of abstraction
It’s a tool not just for chemistry, but for scientific thinking.
Conclusion: Seeing the Unseen
Lewis Dot Structures are more than just a chemical diagram—they are a lens into the unseen world. They bridge the microscopic and the macroscopic, the theoretical and the tangible.
They allow us to draw the invisible. To predict behavior. To understand the matter that makes up our world and our lives.
Whether you’re a student beginning your journey in chemistry or a researcher modeling molecular interactions, Lewis structures offer a map—simple yet profound—of the universe at the atomic level.
And in those dots and lines, we find not just molecules, but meaning.