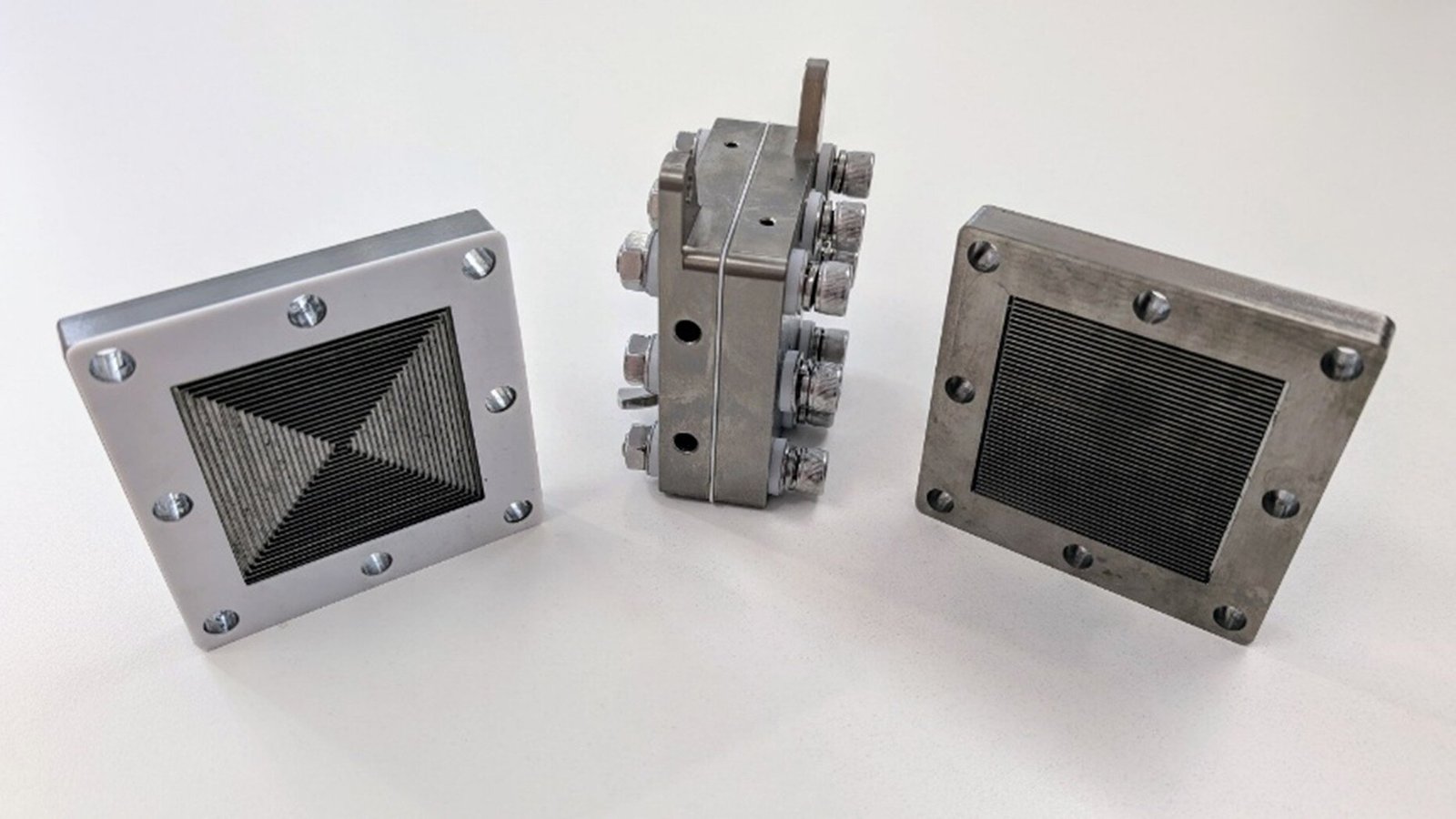
It begins with a spark—one not in the clouds, but in a lab. Not thunder booming across the sky, but a silent, invisible storm humming through a silver box no bigger than a suitcase. And yet, what emerges from that box may just be one of the most profound chemical revolutions of our time.
At the University of Sydney, a team of scientists has created human-made lightning to solve one of the planet’s most urgent conundrums: how to produce ammonia—an unassuming gas that quietly underpins life as we know it—without setting the Earth on fire.
Their breakthrough, published in the journal Angewandte Chemie International Edition, could reshape not only how we grow food but how we power the planet.
A Molecule That Feeds Billions—and Warms the Planet
Ammonia (NH₃) may not be a household name, but it’s everywhere. It’s the cornerstone of fertilizers that feed over half of the world’s population. Without it, the green revolutions of the 20th century would have withered before they ever began. It’s why your dinner exists.
But it comes at a steep cost.
More than 90% of all ammonia today is made using a 120-year-old technique called the Haber-Bosch process—a chemical reaction that combines nitrogen and hydrogen at blistering temperatures and crushing pressures. It requires fossil fuels, especially natural gas, to supply hydrogen. The result? A colossal carbon footprint.
If you measure global industries by their greenhouse gas emissions, ammonia production would rank among the biggest polluters—responsible for nearly 2% of global CO₂ emissions. And yet, demand continues to rise.
“It’s been called the chemical that fed the world,” said Professor PJ Cullen, lead researcher and chemical engineer at the University of Sydney. “But if we want to keep feeding the world without cooking it, we need a better way.”
A Storm in a Box
That “better way” may have just arrived—disguised as lightning.
For six years, Professor Cullen and his team have been chasing a radical vision: to make “green ammonia” using nothing more than air and electricity. Not by scaling up, but by scaling smart. Their method is decentralized, scalable, and, crucially, carbon-free.
Instead of burning gas, they use plasma—a state of matter created by electrifying air. Plasma is what lightning is made of. It’s a chaotic soup of excited particles with immense energy, capable of breaking apart molecules and forging new ones.
“We’re essentially mimicking lightning in a controlled way,” Cullen explained. “It’s not the brute force of Haber-Bosch. It’s more like a dance.”
Here’s how it works:
First, nitrogen and oxygen from the air are zapped into an excited, reactive state using plasma. These molecules are then funneled into a device called a membrane-based electrolyzer—a sleek, silvery box that quietly works the alchemy of turning energy and air into ammonia gas.
The result? Ammonia produced directly in its gaseous form—unlike earlier attempts that created ammonium (NH₄⁺) in liquid, which required additional energy to convert into usable gas.
“This is one of the simplest and most efficient pathways to date for making green ammonia,” Cullen said. “It’s not just a lab curiosity. It’s a potential foundation for future ammonia production.”
Rethinking the Chemical Blueprint of Civilization
To understand just how transformative this could be, consider this: the Haber-Bosch process requires enormous centralized factories, long pipelines, and fossil fuels. It cannot operate without cheap natural gas and industrial-scale infrastructure. That means production is tied to geopolitics, carbon emissions, and massive energy consumption.
Cullen’s plasma-based method, by contrast, is nimble. It can be deployed almost anywhere. It doesn’t need fossil fuels. It can run on renewables. In theory, a single device the size of a refrigerator could sit on a farm and make fertilizer from thin air.
This decentralization is more than just convenience—it’s resilience. In a world increasingly vulnerable to climate shocks and supply chain disruptions, localized ammonia production could mean food security for communities cut off from global fertilizer markets.
And the potential doesn’t end at agriculture.
Ammonia: The Unexpected Star of the Hydrogen Economy
Ammonia contains hydrogen—three hydrogen atoms for every one nitrogen. That makes it an ideal carrier of hydrogen fuel, which is notoriously hard to store and transport. Hydrogen, after all, is the smallest molecule in the universe and loves to leak out of containers.
But turn hydrogen into ammonia, and suddenly it’s easier to ship, store, and use. Crack it back into hydrogen at the destination, and you’ve got a mobile energy source.
This has caught the attention of industries looking to decarbonize—especially shipping, which contributes about 3% of global greenhouse gas emissions. Some experts believe ammonia could replace dirty marine fuels and help ships sail cleaner seas.
So, the silver box in Cullen’s lab is more than a chemical shortcut—it could become a linchpin in the emerging green energy landscape.
The Path Ahead
Of course, challenges remain. The team’s breakthrough is a huge leap, but not the finish line. Right now, the plasma component is energy-efficient and scalable. The next hurdle? Making the electrolyzer just as efficient.
“We’re working on optimizing the second step,” Cullen said. “Our goal is to make the entire process competitive with Haber-Bosch, not just cleaner.”
That means reducing energy consumption, improving yields, and proving reliability at larger scales. But the momentum is there. The method is modular, making it easier to iterate and improve. And with the world urgently looking for ways to decarbonize, support for innovations like this is growing.
From War to Wonder
The irony is hard to ignore. The original Haber-Bosch process didn’t just feed billions—it also enabled the mass production of explosives during both world wars. It was born of both genius and darkness.
Now, over a century later, ammonia might be reborn—not as a harbinger of destruction, but as a quiet savior.
From plasma dancing through air to crops growing in the soil, the loop could finally close. Nature has always made ammonia this way—lightning striking the sky, feeding the earth. Now, human hands have learned the dance.
And in the hum of a machine smaller than a suitcase, a storm begins again—not to destroy, but to nourish, to power, and perhaps even to heal.
Reference: Wanping Xu et al, Regulating Multifunctional Oxygen Vacancies for Plasma‐Driven Air‐to‐Ammonia Conversion, Angewandte Chemie International Edition (2025). DOI: 10.1002/anie.202508240