In a world where clean water is becoming harder to protect, scientists at the University of Adelaide have developed a material that may finally break the grip of one of our most persistent environmental threats — per- and polyfluoroalkyl substances, better known as PFAS. The discovery, published in the journal Small, shows how a light-activated catalyst can completely degrade these so-called “forever chemicals” into harmless byproducts, including fluoride.
This advance offers a potential low-energy, scalable solution for PFAS contamination — a problem that has resisted decades of clean-up efforts. “PFAS contamination continues to pose a global health risk, and this research represents a critical step toward safer communities and cleaner ecosystems,” says lead researcher Dr. Cameron Shearer.
The Challenge of Breaking the Unbreakable
PFAS chemicals are found almost everywhere — in non-stick frying pans, waterproof jackets, firefighting foams, and countless consumer goods. Their unique chemical structure, built on some of the strongest carbon-fluorine bonds known to science, makes them incredibly resistant to breakdown. This durability is why they’ve been so popular in manufacturing — and why they linger in soil, water, wildlife, and even the human bloodstream.
“Many water contaminants are degraded by adding a reactive chemical that binds to the carbon,” Dr. Shearer explains. “But in PFAS molecules, the carbon atoms are protected in such a way that makes this process nearly impossible.”
That chemical armor has made PFAS one of the most stubborn environmental challenges of our time. The result is a creeping accumulation in ecosystems and human bodies, linked to developmental problems, fertility issues, immune suppression, and cancer.
Recent studies reveal that over 85% of Australians have detectable PFAS in their blood, and new drinking water standards now limit some PFAS compounds to just a few nanograms per liter — a testament to the risks they pose even at ultra-low concentrations.
Harnessing the Power of Sunlight
The Adelaide team took a different approach: rather than trying to rip apart PFAS with brute chemical force, they designed a catalyst that uses light itself to do the work. Under sunlight, the material produces highly reactive species that target the fluorine atoms protecting PFAS molecules. Once those bonds are broken, the rest of the molecule quickly falls apart.
The process doesn’t just destroy PFAS — it transforms them into benign components. One of these is fluoride, a compound already familiar in small doses from toothpaste and dental care, and which can also be used as an agricultural additive.
“We’ve altered conditions and optimized the catalyst to target the PFAS-protective F atoms, which resulted in complete breakdown of the forever chemicals,” says Dr. Shearer. “The produced fluoride can be isolated and reused, turning a pollutant into a resource.”
Toward Large-Scale Clean-Up
While the research is still in its early stages, the potential is significant. Dr. Shearer envisions the new material as part of a multi-step PFAS treatment chain. First, contaminated water would be filtered to concentrate PFAS into a smaller volume. That concentrated waste could then be exposed to the light-activated material, breaking the molecules apart and neutralizing their environmental impact.
“This is a proof-of-concept,” Dr. Shearer notes. “Our next step is to improve the stability of the materials so they can survive and perform under large-scale water treatment conditions. That work is already underway, led by my University of Adelaide colleague, Mahmoud Gharib.”
If successful, the technology could bring PFAS remediation out of specialized labs and into practical, low-cost water treatment plants, helping communities around the world reclaim their drinking water from this invisible but dangerous threat.
A Path to a Cleaner Future
PFAS have been a chemical success story for industry — and a slow-burning disaster for the planet. Their near-indestructibility has meant they’ve traveled far beyond the factories where they were made, showing up in Arctic ice, deep ocean waters, and human bloodstreams everywhere.
The University of Adelaide’s discovery offers a rare kind of hope in environmental science: the chance not just to contain pollution, but to undo it. By enlisting the free, renewable power of sunlight, it points toward a cleaner, safer, and more sustainable future.
For now, PFAS may still be called “forever chemicals.” But thanks to research like this, their forever might finally have an expiration date.
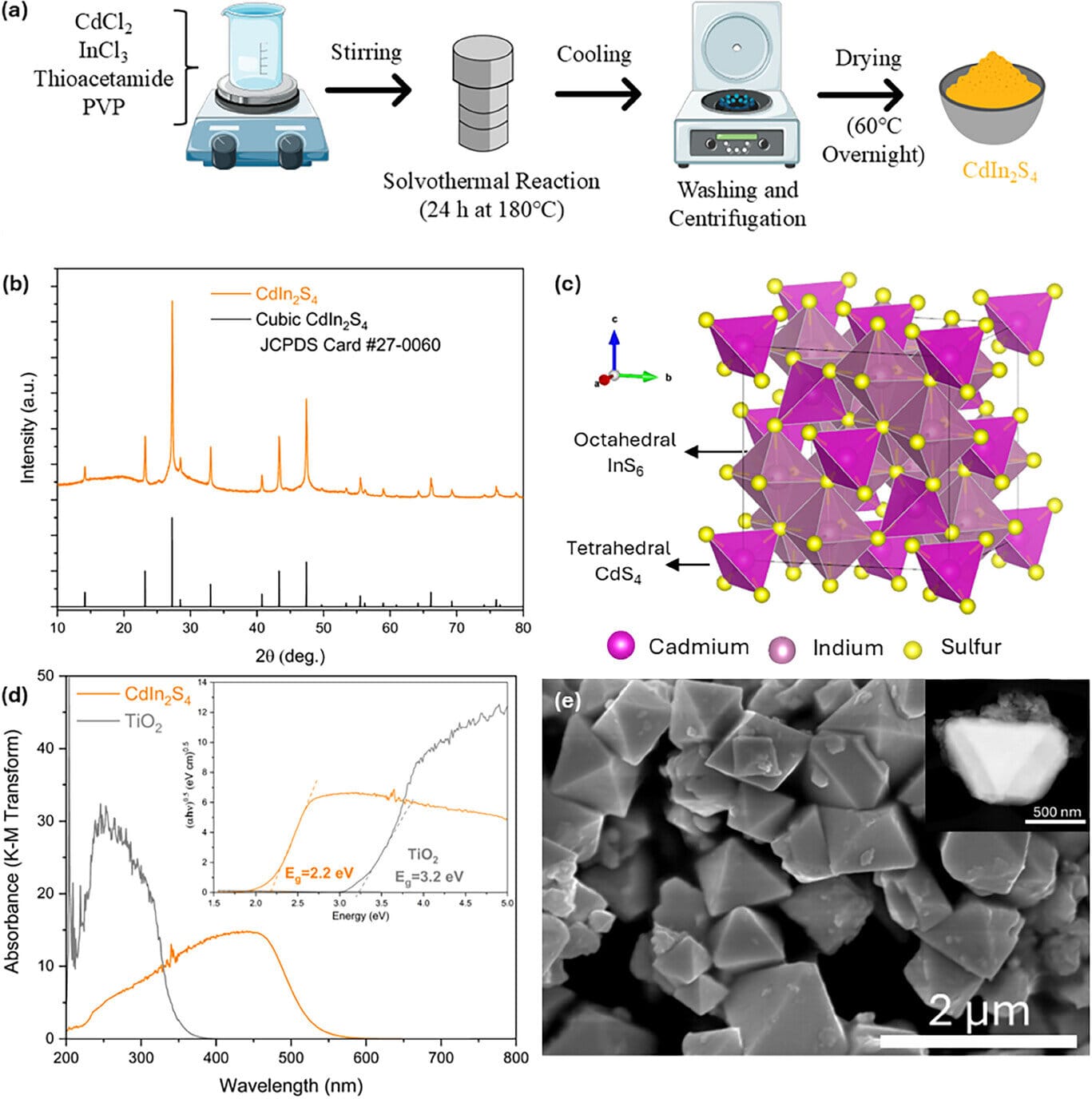
More information: Mahmoud Adel Hamza et al, CdIn2S4 Micro‐Pyramids for Reductive Photocatalytic Degradation of Perfluorooctanesulfonic Acid, Small (2025). DOI: 10.1002/smll.202504601