Every breath we take, every step we walk, every bite of food we eat is powered by chemistry. Yet most of us rarely stop to notice the invisible reactions happening around—and inside—us every second. From the fizz of soda to the rust on an old gate, from the smell of fresh bread to the burst of flavor in a ripe orange, chemistry is quietly orchestrating our experiences. It is the unseen artist painting the world with color, taste, and energy.
Chemistry often feels like something confined to laboratories and complex formulas. But step back and look closely: the kitchen is a chemical workshop, the bathroom a miniature lab, the outdoors a vast chemical playground. Understanding these everyday reactions doesn’t just explain the world—it reveals its wonder. It turns routine moments into small miracles and shows that the science of atoms and molecules isn’t distant but deeply woven into the fabric of life.
Breathing: The First Chemical Dance
Before we taste food or feel sunlight, our bodies are already performing one of nature’s most elegant chemical reactions: breathing. Each inhale pulls in oxygen molecules (O₂), invisible but vital. Deep inside our lungs, these molecules pass into tiny sacs where they meet red blood cells. Here, hemoglobin proteins—with their iron-rich centers—bind oxygen in a dance of molecular attraction.
As oxygen travels through the bloodstream, it fuels cellular respiration, a process that combines oxygen with glucose (C₆H₁₂O₆) to release energy. The equation is deceptively simple:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + energy (ATP)
But this reaction is life itself. It powers every heartbeat, every thought, every moment of movement. The carbon dioxide (CO₂) produced is exhaled, ready to nourish plants that will, in turn, release more oxygen. In every breath, we are part of an endless cycle—a global chemical reaction linking every creature on Earth.
Cooking: Transforming Matter with Heat
Step into a kitchen, and you step into a chemistry lab where transformations happen every minute. When butter melts on a hot pan, heat energy breaks intermolecular forces, turning solid into liquid. When water boils, molecules escape into gas form. But cooking is more than phase changes; it’s a cascade of complex chemical reactions that create flavor and aroma.
The browning of bread, steak, or roasted coffee is driven by the Maillard reaction—a dance between amino acids and sugars triggered by heat. This reaction produces hundreds of flavor compounds, giving us the nutty, savory taste of grilled food and the comforting smell of toasted bread. Caramelization, another heat-driven reaction, breaks down sugars into golden syrups with rich sweetness.
Even something as simple as frying an egg is a chemical marvel. Heat denatures the proteins in egg whites, unraveling their shapes so they can link together into a new solid structure. This is why transparent egg whites turn opaque and firm—a visible sign of chemistry reshaping molecules.
Taste and Smell: The Chemistry of Senses
Our enjoyment of food, perfume, or flowers is tied to chemistry at a molecular level. Taste buds detect five primary flavors—sweet, sour, salty, bitter, and umami—each linked to specific chemical compounds. Sweetness comes from sugars binding to taste receptors; sourness is triggered by acids releasing hydrogen ions (H⁺); bitterness often signals plant alkaloids, some of which are toxic in large amounts; umami arises from glutamates, abundant in cheese, meat, and soy sauce.
Smell is even more sensitive. Tiny molecules drift into our noses and bind to olfactory receptors, each tuned to a particular chemical shape. The aroma of fresh-cut grass is caused by green leaf volatiles—aldehydes and alcohols released when plants are injured. The smell of rain comes from geosmin, an earthy compound produced by soil bacteria. Our memories attach strongly to scents because these chemical signals travel directly to the brain’s limbic system, where emotions and memory are deeply rooted.
Soap and Cleaning: Chemistry Against Dirt
When you wash your hands, scrub dishes, or clean clothes, you rely on chemical reactions to lift away grime. Dirt and grease cling to surfaces because they are hydrophobic—they repel water. Soap molecules have a unique structure with two ends: a hydrophobic tail that grabs onto oils and a hydrophilic head that loves water.
When soap is mixed with water, these molecules form micelles—tiny spherical structures that trap oil and dirt inside while the water-loving heads stay outside. With rinsing, micelles wash away, taking dirt along. Detergents and shampoos work on the same principle, but often include enzymes that break down proteins and fats chemically, making cleaning even more effective.
Disinfectants, meanwhile, use chemical agents like alcohol or chlorine to disrupt the cell membranes of bacteria and viruses, killing harmful microbes. Without realizing it, every time we clean, we’re performing precise chemical reactions designed to protect health.
Rust and Corrosion: Metal’s Slow Dance with Oxygen
Look at an old bicycle or a weathered fence, and you’ll see chemistry’s slow handiwork—rust. When iron is exposed to oxygen and moisture, it undergoes oxidation, forming iron oxide (Fe₂O₃). This reddish-brown compound flakes off, weakening the metal. The reaction is simple but relentless:
4Fe + 3O₂ + 6H₂O → 4Fe(OH)₃ → Fe₂O₃·xH₂O
Rust is not just decay; it’s a transformation driven by environmental chemistry. Protective coatings, such as paint or galvanization with zinc, work by blocking oxygen or sacrificing a more reactive metal to corrode first. Without these measures, metal structures—from bridges to ships—would crumble far faster, showing how everyday engineering is rooted in understanding chemical reactions.
Combustion: The Fire That Powers Civilization
Strike a match, light a stove, start a car engine—all these acts unleash combustion, one of the most important chemical reactions for human progress. Combustion is the rapid oxidation of a fuel, producing heat, light, and gases. The fire we see is energy released as bonds in molecules break and reform.
For instance, burning wood involves cellulose reacting with oxygen:
C₆H₁₀O₅ + 6O₂ → 6CO₂ + 5H₂O + heat
In cars, gasoline undergoes a similar reaction inside engines, releasing energy that pushes pistons and moves wheels. Controlled combustion has powered homes, industries, and entire economies for centuries. But incomplete combustion can produce harmful carbon monoxide (CO) and particulates, highlighting the delicate chemistry between fuel, oxygen, and heat.
Photosynthesis and Everyday Green
While combustion releases energy, photosynthesis stores it—a chemical miracle happening quietly in every green leaf. Plants absorb sunlight, water, and carbon dioxide to synthesize glucose and oxygen:
6CO₂ + 6H₂O + light → C₆H₁₂O₆ + 6O₂
This process not only feeds plants but fuels nearly every ecosystem on Earth. The oxygen we breathe and the food we eat are products of this ancient chemical reaction. Even the wood we burn and the fossil fuels that power cities trace their origins to sunlight captured by plants millions of years ago. Chemistry is thus the thread linking light to life itself.
Acid-Base Reactions in the Kitchen
Open a bottle of vinegar and you release acetic acid’s sharp tang. Add baking soda (a base) and watch it fizz as carbon dioxide forms. This is a simple acid-base reaction:
NaHCO₃ + CH₃COOH → CO₂ + H₂O + NaCH₃COO
Such reactions are everywhere in cooking. Lemon juice tenderizes meat by breaking down proteins with citric acid. Baking powder leavens cakes through acid-base chemistry that releases gas bubbles. Even the sour taste in yogurt comes from lactic acid produced when bacteria ferment milk sugars.
Inside our bodies, acid-base chemistry keeps blood at a stable pH of around 7.4. Buffers—mixtures of weak acids and bases—counter sudden changes, ensuring enzymes can function properly. Without these invisible reactions, life would collapse in moments.
Everyday Oxidation and Reduction
Not all chemical reactions involve flames or fizz. Oxidation and reduction (redox) quietly govern countless transformations. Browning apples and bananas, for example, results from oxidation when exposed to air. Antioxidants like vitamin C slow this process by donating electrons, preventing discoloration.
In batteries, redox reactions convert chemical energy into electricity. When you switch on a flashlight, electrons flow through a circuit because one material gives up electrons (oxidation) while another accepts them (reduction). From smartphones to cars, nearly every device we use depends on controlling these reactions safely and efficiently.
Fermentation: Microbial Alchemy
Bread, cheese, wine, and beer share a common origin: fermentation, a chemical process driven by microorganisms. Yeast converts sugars into alcohol and carbon dioxide:
C₆H₁₂O₆ → 2C₂H₅OH + 2CO₂
This reaction makes bread rise and beverages bubble. In yogurt and sauerkraut, bacteria produce lactic acid, giving tangy flavors and preserving food naturally. These processes are among humanity’s oldest uses of chemistry, discovered long before scientists understood the molecular details. Today, fermentation also creates biofuels, pharmaceuticals, and sustainable materials, proving its enduring importance.
The Chemistry of Cleaning the Air
Invisible reactions also purify the environment. Plants remove carbon dioxide and release oxygen, but air itself cleanses through chemical interactions. Ozone in the upper atmosphere absorbs harmful ultraviolet radiation. Hydroxyl radicals, formed when sunlight splits water vapor, react with pollutants, breaking them down naturally.
Even rain has chemistry—it washes particulates from the air and carries dissolved nitrogen compounds that fertilize soil. Yet human activity can overwhelm these natural processes, producing smog and acid rain. Understanding atmospheric chemistry helps design solutions, from catalytic converters in cars to advanced air filters that capture harmful molecules.
Emotional Chemistry: Neurotransmitters and Feelings
Chemistry doesn’t just shape the physical world; it molds our emotions and thoughts. The brain communicates through neurotransmitters—chemical messengers that pass signals between neurons. Dopamine triggers pleasure and reward sensations. Serotonin regulates mood and well-being. Adrenaline readies us for action during stress.
Love, fear, happiness—all are grounded in chemical reactions involving complex pathways of hormones and neurotransmitters. When you fall in love, oxytocin and dopamine surge, creating feelings of attachment and euphoria. Laughter releases endorphins, natural painkillers that lift spirits.
Medications for anxiety or depression often work by altering these chemical balances, showing how deeply brain chemistry shapes human experience. Understanding these reactions not only explains behavior but opens doors to improving mental health and emotional resilience.
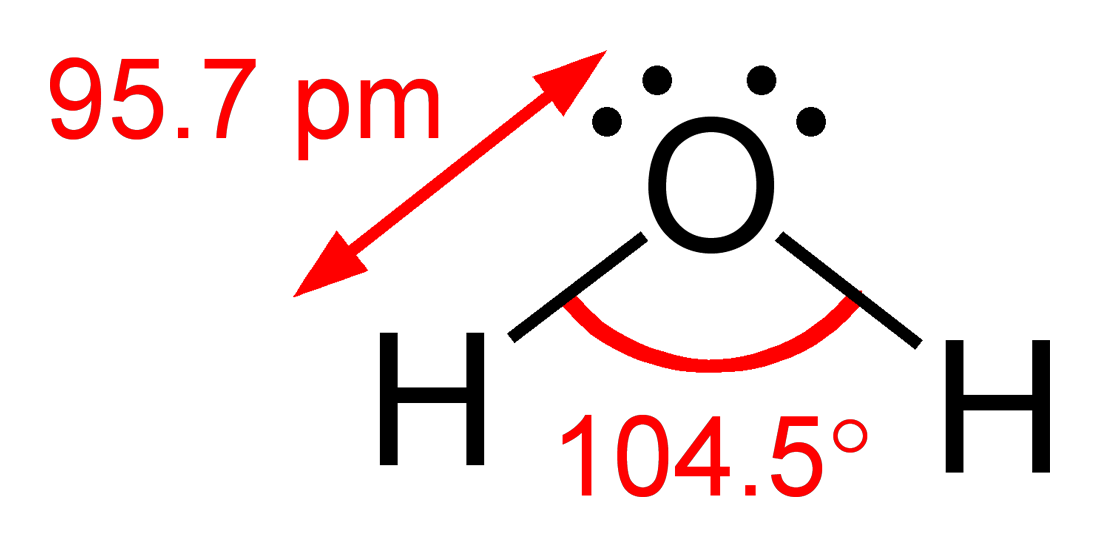
Water: The Universal Solvent of Life
Life’s chemistry is impossible without water. Its molecular structure—two hydrogens and one oxygen—gives it unique properties. Water dissolves salts and nutrients, enabling countless reactions inside cells. It moderates temperature by absorbing heat without major temperature swings.
When you add sugar to tea, water molecules surround sugar crystals, pulling them apart and dispersing them evenly. Detergents work because water’s polarity allows soap molecules to interact with both oil and water, washing dirt away. Even digestion relies on water breaking down complex molecules into nutrients the body can absorb.
Chemically, water participates in hydrolysis reactions that split compounds and condensation reactions that build them. It is not just a passive medium but an active player in life’s chemistry, present in every reaction that sustains us.
Light and Color: A Dance of Molecules
The vibrant colors of sunsets, flowers, and even clothing stem from chemical interactions with light. Pigments absorb specific wavelengths of light and reflect others, creating visible colors. Chlorophyll makes plants green by absorbing red and blue light for photosynthesis. Carotenoids produce oranges and yellows in autumn leaves.
In synthetic dyes, carefully engineered molecules interact with light to create stable, vivid colors for fabrics and inks. Even the screen you’re reading this on uses light-emitting diodes (LEDs), which rely on semiconductors and quantum chemical principles to produce colors.
Fireworks offer a spectacular example: metal salts heated in explosions release energy as colored light—strontium gives red, barium green, copper blue. Each dazzling display is a chemical fingerprint burning against the night sky.
The Chemistry of Health and Medicine
Every pill we take, every vaccine we receive, is chemistry crafted to heal. Pain relievers like aspirin work by inhibiting enzymes involved in producing prostaglandins—chemicals that signal pain and inflammation. Antibiotics target bacterial cell walls or protein synthesis, stopping infections at a molecular level.
In our bodies, enzymes—biological catalysts—speed up reactions essential for life. Digestive enzymes break down food. DNA polymerases copy genetic information. Hormones like insulin regulate blood sugar through signaling pathways.
Even the simple act of drinking water or eating fruit introduces chemicals that maintain health: electrolytes like sodium and potassium balance nerve function; antioxidants neutralize harmful free radicals. Medicine and nutrition are not separate from chemistry—they are its applied wisdom for human survival.
The Unseen Reactions That Shape the Future
Chemistry is not just part of the past or present; it is shaping the future of humanity. Renewable energy technologies, from solar panels to hydrogen fuel cells, rely on advanced chemical reactions to store and release energy cleanly. Green chemistry seeks to design processes that reduce waste and avoid toxic substances, paving the way for sustainable industries.
Nanotechnology manipulates matter at the atomic scale, creating materials with novel properties for medicine, electronics, and environmental protection. Biochemistry and genetic engineering enable precise control of reactions inside living organisms, offering breakthroughs in curing diseases and feeding the world.
Yet at its core, the chemistry of tomorrow remains rooted in the reactions we witness daily—in cooking, cleaning, breathing, and building. Understanding these simple reactions connects us to a greater vision of how chemistry can solve the challenges ahead.
A World Alive with Chemistry
From dawn to dusk, chemistry is at play in ways we often overlook. The smell of morning coffee, the warmth of sunlight on skin, the sparkle of clean glass, the rustle of leaves—all are born of molecules in motion, bonds breaking and forming, energy flowing invisibly through matter.
Chemistry is not confined to test tubes and laboratories. It lives in kitchens, gardens, streets, and our own bodies. Every reaction, no matter how small, is part of a grander story of transformation and connection.
When we recognize these everyday reactions, the world changes. Soap bubbles become marvels of surface tension and molecular attraction. A candle flame becomes a miniature sun, converting wax into light and warmth. Even the simple act of breathing becomes a profound chemical bond linking us to the entire living planet.
To see life through the lens of chemistry is to see it anew—not as mundane routines, but as an intricate, beautiful, and endlessly creative process written in the language of molecules. It is to realize that we are not just living in a world shaped by chemistry—we are chemistry itself, walking, thinking, and dreaming in the wonder of reactions all around us.