In the race to build a cleaner, more sustainable future, hydrogen is often hailed as a game-changer. Unlike fossil fuels, hydrogen burns cleanly, releasing only water as a byproduct. But not all hydrogen is created equal. The most promising form—green hydrogen—comes from splitting water using renewable electricity. Yet, making this process efficient has remained one of the great scientific challenges of our time.
Now, researchers from the Fritz Haber Institute of the Max Planck Society have uncovered new details about what really happens during this critical reaction. Their work, published in Nature Chemistry, provides a window into the hidden world at the interface between catalysts and water. What they’ve found could help transform the future of energy.
The Bottleneck in Hydrogen Production
At the heart of water splitting lies a stubborn obstacle known as the oxygen evolution reaction (OER). While hydrogen atoms split off easily, oxygen requires a more complex set of steps, and this slows everything down. If OER could be accelerated, hydrogen could be produced more efficiently and at larger scales, bringing us closer to a renewable energy economy.
Catalysts—materials that speed up chemical reactions without being consumed—are key players here. But even the best catalysts struggle when it comes to OER. The question has long been: why?
A Fresh Look at Catalysts in Action
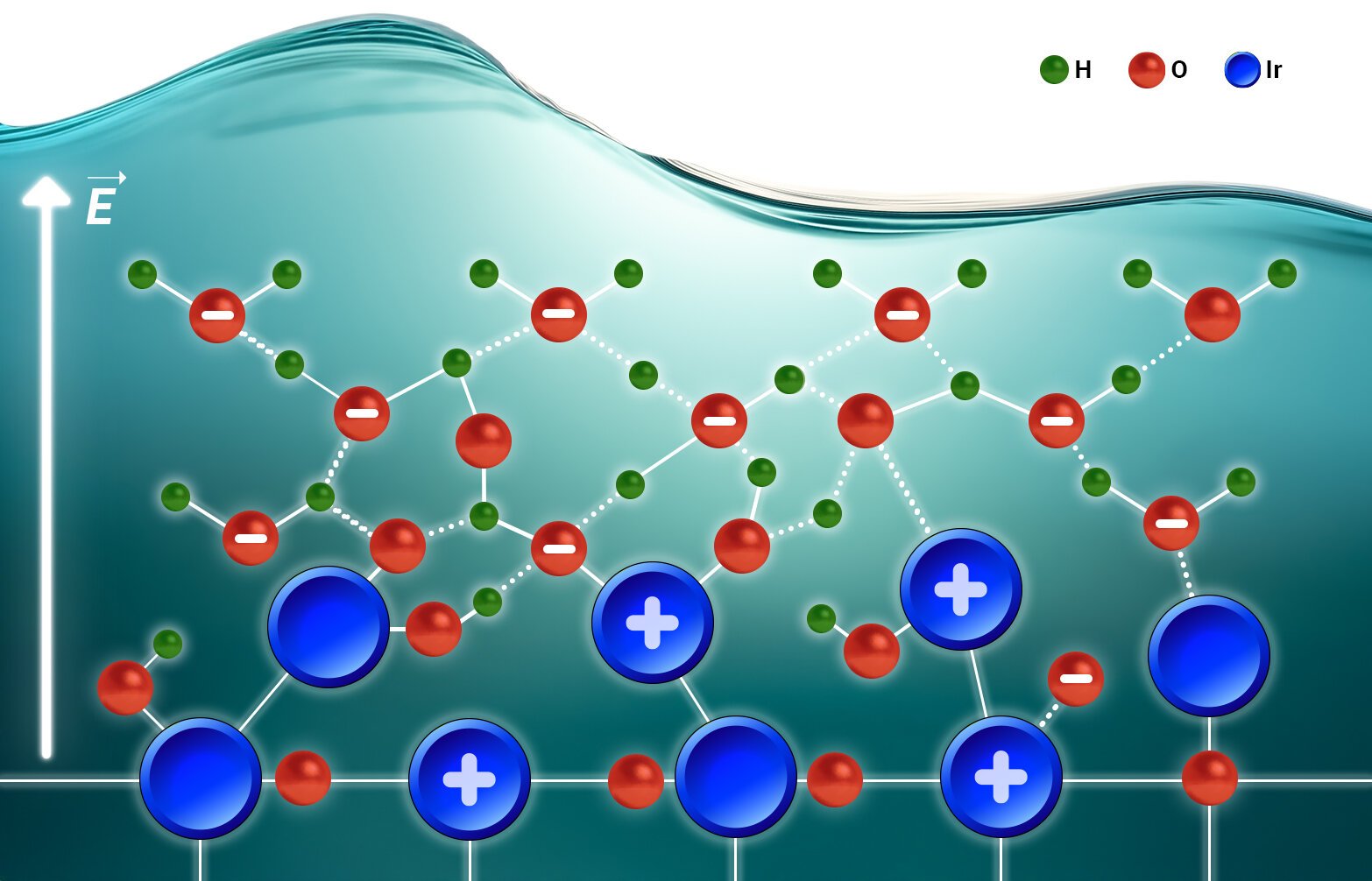
Led by Dr. Martinez-Hincapié and Dr. Oener, the team at the Department of Interface Science decided to peer directly into the process. Using a combination of temperature-dependent electrochemistry and operando spectroscopy, they observed the catalysts not in idealized lab conditions but in the thick of action, immersed in water, under realistic operating potentials.
What they discovered is that the secret lies not only in the catalyst itself, but also in the way it interacts with its watery environment. The solvent—the surrounding water molecules—plays a surprisingly active role, and the reaction cannot be fully understood by looking at the solid surface alone.
The Dance of Charge and Solvent
The team identified a striking transition point in the reaction. At low bias, the catalyst is sluggish, held back by an accumulation of excess charge. But at a certain potential, something changes: the excess charge no longer limits the process, and the catalyst becomes dramatically more active.
The reason comes down to a phenomenon known as interfacial solvation. Ions at the interface gain or lose solvent molecules, reshaping the environment around the reaction. This exchange, though subtle, is pivotal. It determines how charges are stabilized on the catalyst and how effectively the reaction can proceed.
As Dr. Oener put it, “We should really think about the catalyst-electrolyte interphase as a whole, not in separate terms. The solid surface and the surrounding solvent are in constant conversation, and their interplay is what drives the activity we observe.”
A Catalyst’s Hidden Transformations
Beyond the role of solvent, the team also observed how the catalyst itself changes during the reaction. Using operando X-ray spectroscopy, they captured the oxide surface adapting both structurally and chemically right at the crucial transition point.
This adaptation is not a superficial effect, nor is it tied to how much catalyst is used or its surface area. Instead, it reveals something intrinsic: the true activity comes from the catalyst’s ability to hold and redistribute charge in coordination with the solvated ions. In other words, efficiency is not just about the material but about how it evolves in harmony with the liquid environment.
Why This Matters for the Energy Transition
The implications of these findings are profound. Understanding catalysts in such detail paves the way for designing new materials that are not just marginally better but fundamentally more efficient. If we can tailor catalysts to optimize this interplay between surface and solvent, we may unlock a step-change in green hydrogen production.
Professor Beatriz Roldán Cuenya emphasized the importance of combining different operando spectro-microscopy techniques to gain these insights. By probing both the solid catalyst and the liquid environment simultaneously, her team has pieced together a fuller, more dynamic picture of what really happens at the interface.
Beyond the Lab: Toward Real-World Solutions
Science of this kind may sound abstract, but its potential impact is concrete and far-reaching. Green hydrogen is seen as a cornerstone of future energy systems, capable of powering industries, fueling transportation, and storing renewable energy at scale. However, its widespread adoption hinges on solving efficiency challenges like the oxygen evolution reaction.
This research brings us closer to that goal. By revealing how catalysts and water molecules “dance” together during electrolysis, the Fritz Haber Institute team has shown us that the pathway to cleaner energy lies not only in developing better materials but also in understanding the subtle choreography of matter itself.
The Road Ahead
The journey is far from over. Many mysteries remain about the intricate relationship between catalysts and solvents. But with each discovery, the picture becomes clearer, and the promise of scalable, affordable green hydrogen comes closer.
This study is more than a technical achievement—it is a glimpse into a future where the fundamental forces of chemistry and physics are harnessed for humanity’s greatest challenge: powering the world without harming the planet.
The dance between water, charge, and catalyst has always been there, invisible to our eyes. Thanks to the scientists at the Fritz Haber Institute, we are beginning to see its steps more clearly. And with that knowledge, we may one day choreograph a new era of clean energy.
More information: Ricardo Martínez-Hincapié et al, Interfacial solvation pre-organizes the transition state of the oxygen evolution reaction, Nature Chemistry (2025). DOI: 10.1038/s41557-025-01932-7