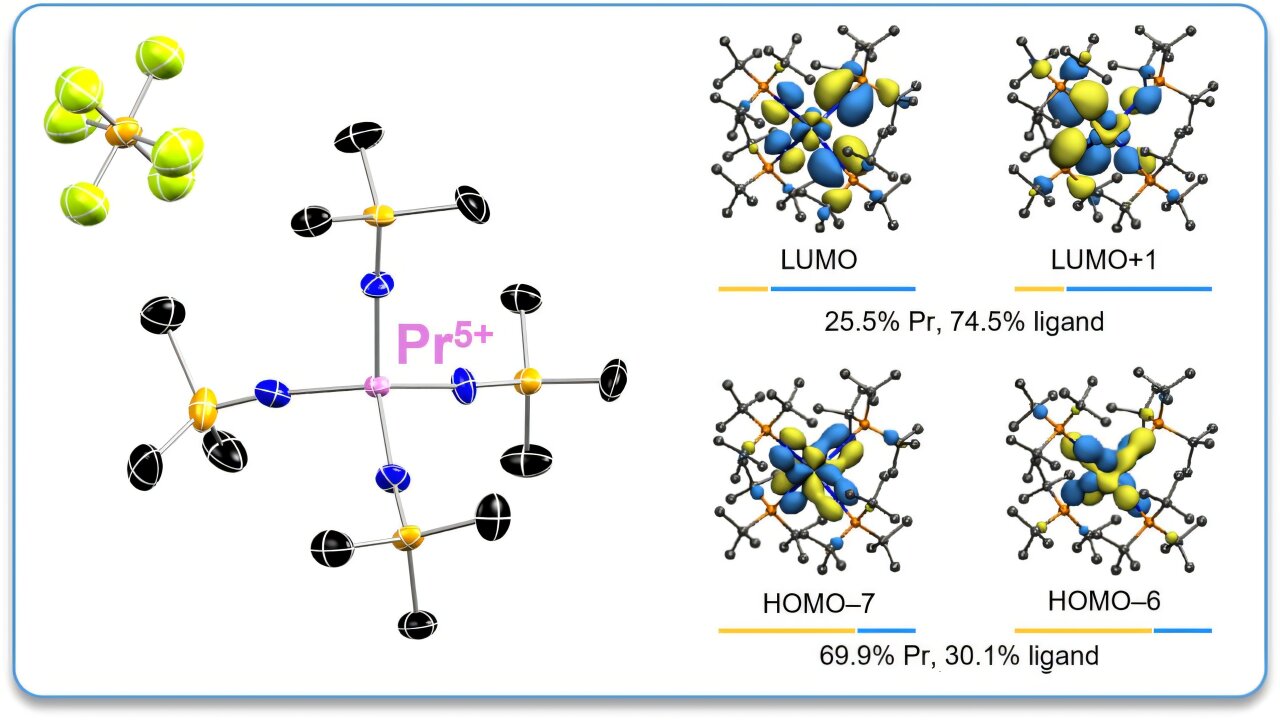
It started with a whisper in the world of chemistry—a suspicion dating back to the 1890s that somewhere within the rare earth elements, there might be untapped powers hidden in plain sight. Now, over a century later, scientists have finally found what others could only imagine: a new oxidation state for one of the most elusive members of the periodic table—praseodymium.
Led by researchers at Georgia Tech, in collaboration with the University of Iowa and Washington State University, this groundbreaking discovery marks the first time a lanthanide element has achieved a +5 oxidation state, a level of chemical reactivity never before witnessed in this family of metals. The implications could reshape our understanding of materials science, open doors to advanced electronics, and give the modern world a much-needed boost in the race to secure critical resources.
Published recently in Nature Chemistry, the study titled “Praseodymium in the Formal +5 Oxidation State” isn’t just about refining chemistry—it’s about redefining what’s possible.
A Rare Glimpse Into Rare Earths
Lanthanides, often referred to as rare earth elements (REEs), are a family of 15 silvery-white metals known for their powerful magnetic, optical, and electronic properties. Though not particularly rare in the Earth’s crust, their chemical similarities make them notoriously difficult to separate and purify, which has long limited how we use them.
Yet our dependence on these elements is enormous. From MRI machines and LED screens to military guidance systems and smartphones, lanthanides make modern life possible. We extract them, use them, discard them—often without ever realizing they were there. But we’ve only been using them in one specific way, primarily through their +3 oxidation state, the stable form that governs how these metals behave in most technologies.
Until now, that was the limit.
“We only use them commercially in one oxidation state—the 3+ oxidation state—which defines a set of magnetic and optical properties,” said Dr. Henry “Pete” La Pierre, associate professor at Georgia Tech and corresponding author of the study. “If you can stabilize a higher oxidation state, it could lead to entirely new magnetic and optical properties.”
In other words, if chemistry were music, we’ve only ever heard one key from the lanthanides. Now we’ve stumbled upon a new octave.
Like Discovering a New Element
Oxidation, the process by which an atom loses electrons, may sound mundane—but it’s the quiet engine behind much of what drives the natural and technological world. It’s what causes apples to brown and metal to rust, but it’s also responsible for batteries, combustion, corrosion, and even some catalytic processes in industrial chemistry.
In praseodymium’s case, achieving a +5 oxidation state is like pulling a ghost out of the periodic table. Chemists had long predicted its existence but lacked the tools to stabilize or observe it—until now.
“This is like discovering a new element,” La Pierre explained. “A new oxidation state tells us what we don’t know and gives us ideas for where to go.”
The team was able to isolate praseodymium in its +5 state using precision synthesis and ultra-sensitive detection tools. This wasn’t just about adding a few chemicals and seeing what sticks. It took years of theorizing, experimentation, and collaboration to capture something so inherently unstable and ephemeral.
Their success reveals new chemical behavior for the element and expands the realm of possibilities for the entire lanthanide series.
From Lab Bench to the Real World
This isn’t just an academic curiosity—it’s a technological catalyst. If scientists can now explore and harness the higher oxidation states of lanthanides, the implications reach far beyond a few chemistry textbooks.
The breakthrough may spark innovations in quantum technology, optical sensors, advanced electronics, and even next-generation computing devices. With each oxidation state comes a unique set of magnetic and optical traits, like new instruments in a symphony. In fields where precision and performance matter—especially where miniaturization and power-efficiency are key—this could be a game-changer.
But there’s another urgent reason why this discovery matters: the global race for rare earth elements is intensifying.
Breaking the Supply Chain Barrier
Despite their critical importance, REEs are notoriously hard to obtain. They’re often interlocked within the same minerals, making separation slow, costly, and environmentally damaging. The United States, for instance, depends heavily on foreign sources—primarily China—for its supply of these elements.
The separation process, as it stands, is a logistical nightmare: massive quantities of mined material yield tiny amounts of usable elements, producing considerable waste along the way. It’s not just inefficient—it’s unsustainable.
By unlocking new oxidation states, La Pierre and his colleagues hope to create better, cleaner ways to isolate individual lanthanides, potentially revolutionizing how we extract and recycle REEs. “We could improve rare earth separation, reduce waste, and enhance the sustainability of these critical materials,” La Pierre noted.
With oxidation chemistry as the key, the team envisions new tools for not just using rare earths more effectively, but sourcing them in a way that’s less harmful to the planet and less dependent on unstable supply chains.
The Future Hidden in the Table
Scientific revolutions don’t always come with fireworks. Sometimes, they appear in the quiet shimmer of a new number beside an old name on the periodic table. But these small changes can ripple out in massive ways.
By revealing praseodymium’s hidden potential, La Pierre’s team has opened a door into a world of chemical possibilities that were previously off-limits. The periodic table—often seen as fixed and complete—just gained a new dimension.
As research continues, scientists will explore whether other lanthanides can also reach this +5 state, or perhaps even higher. Each success would expand our understanding of matter, energy, and the universe itself.
For now, what began as a theoretical whisper in the 19th century has finally found its voice. And the message is clear: even in the oldest corners of science, there is always something new waiting to be discovered.
Reference: Andrew C. Boggiano et al, Praseodymium in the formal +5 oxidation state, Nature Chemistry (2025). DOI: 10.1038/s41557-025-01797-w