Imagine a future where carbon dioxide—the invisible gas warming our planet—could be captured straight from the air and turned into durable plastic, cleanly and efficiently. No fossil fuels. No forests cut down. Just light, air, water, and a touch of chemistry. That vision, long part of environmental science fiction, is beginning to solidify into reality.
In a breakthrough that could redefine carbon recycling, chemists at the California Institute of Technology (Caltech) have created a working system that transforms carbon dioxide (CO₂) into a strong, versatile plastic known as polyketone, using nothing but electricity, water, and smart engineering. Their results, published in the journal Angewandte Chemie International Edition, could open new doors for turning one of Earth’s most dangerous greenhouse gases into a valuable industrial resource.
Engineering a Synthetic Green Leaf
“This is essentially artificial photosynthesis—but instead of sugars, we’re making plastic,” says Max Zhelyabovskiy, a Caltech graduate student and lead author on the study. “We’re using renewable electricity to mimic the work of plants, but directing that energy toward manufacturing.”
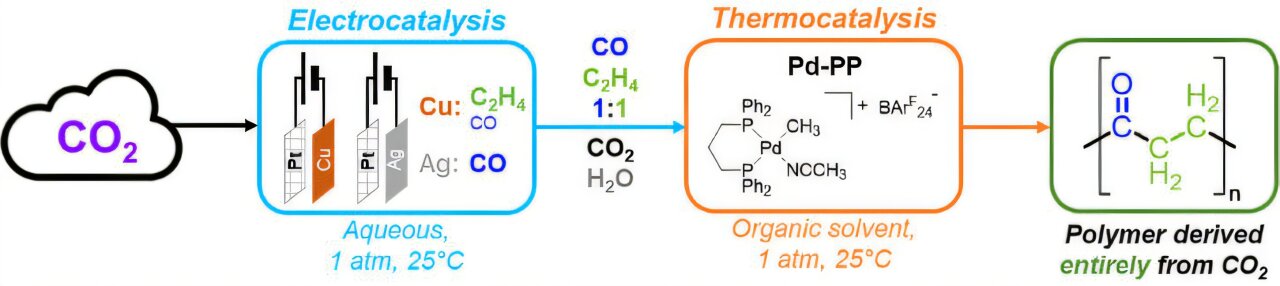
At the core of the process is a dual-loop system—two carefully coordinated chemical environments that each play a vital role in reshaping CO₂ into something far more useful. The first loop converts carbon dioxide into simpler building blocks, while the second combines them into complex plastic molecules.
But what sets the Caltech innovation apart is its efficiency. Previous attempts at such CO₂-to-plastic conversion produced only trace amounts of useful gases like ethylene and carbon monoxide—the ingredients needed to build polymers. This time, however, the team managed to boost those concentrations significantly, with 11% ethylene and 14% carbon monoxide—enough to meaningfully drive the second stage of the reaction forward.
The Plastic Born From Air
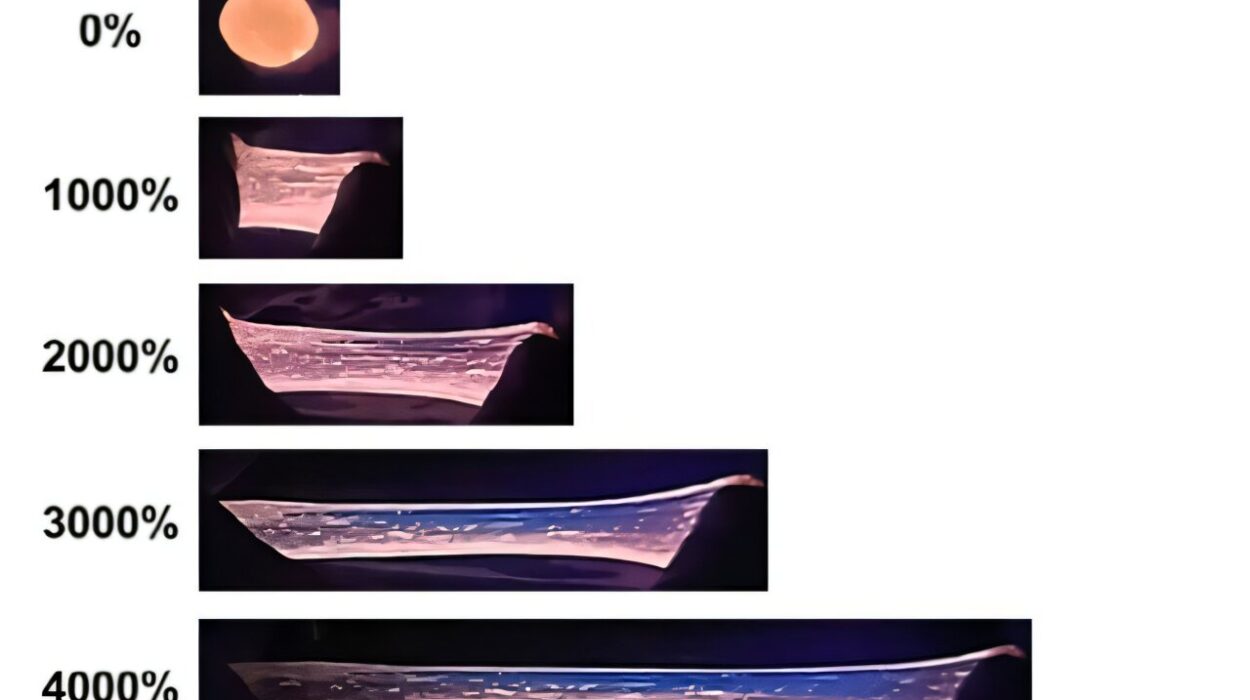
The plastic created in this system, polyketone, is no mere novelty. Known for its mechanical strength, resistance to heat and chemicals, and durability, it’s already used in a wide range of industries—from automotive components and industrial piping to adhesives and even sports gear.
“CO₂ is a problem because we have too much of it,” says Professor Theo Agapie, a senior author on the paper and the John Stauffer Professor of Chemistry at Caltech. “But it’s also a resource—an abundant, inexpensive feedstock. If we can turn it into something valuable using clean electricity, we’re solving two problems at once.”
This dual benefit—removing CO₂ from the atmosphere while replacing petroleum-based plastics—makes the discovery especially compelling. But the road to this achievement wasn’t smooth.
Building a Two-Step Chemical Dance
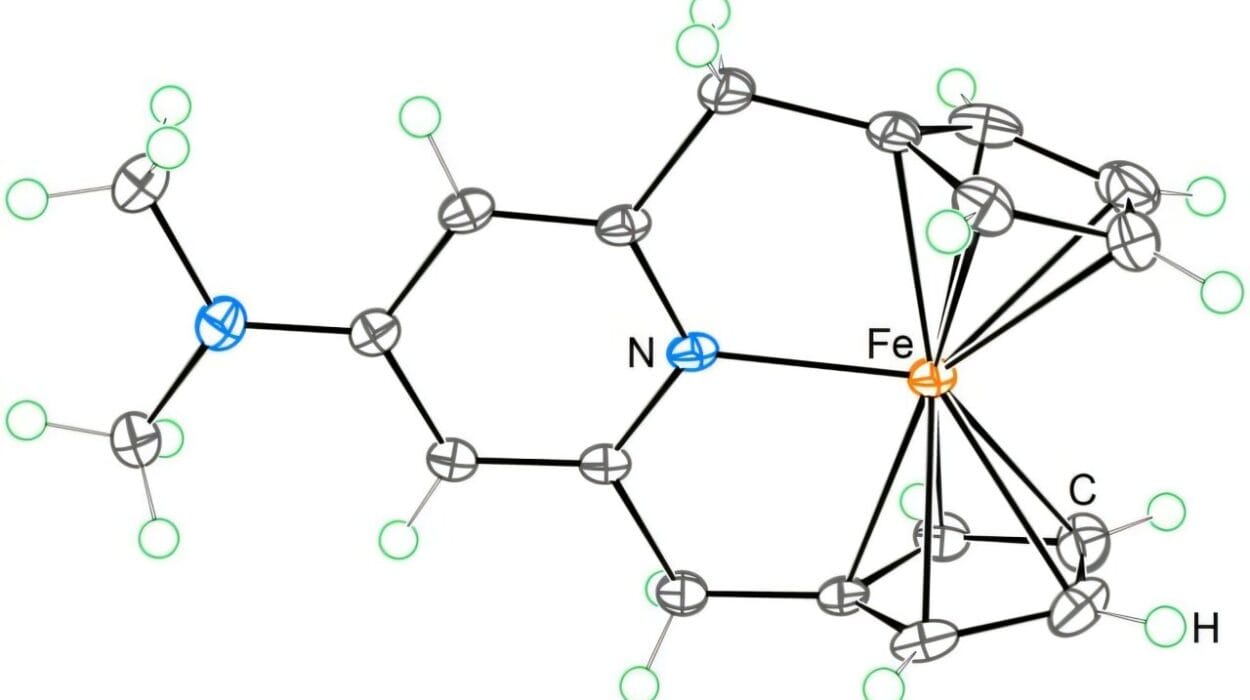
The system developed by the team is elegant in its design. In the first stage, CO₂ is pumped into a gas chamber where it encounters gas diffusion electrodes—specially engineered materials coated with copper. These copper surfaces, combined with a flowing potassium bicarbonate solution and a low voltage of electricity, trigger a reaction that splits CO₂ into ethylene and carbon monoxide.
The gas mixture, still laced with impurities like hydrogen and water vapor, is then pushed into a second reaction chamber. Here, a palladium catalyst takes over, bubbling the gases through a liquid that encourages the monomers to link up into long chains—forming polyketone polymers.
What makes this system extraordinary is its tolerance for “messy” conditions. Real-world chemical reactions are rarely clean, and many polymerization catalysts break down in the presence of moisture or other contaminants. The Caltech team, however, identified a palladium catalyst robust enough to operate even under less-than-perfect conditions.
“This is rare,” Zhelyabovskiy notes. “Most systems only work with ultra-pure ingredients, which limits their practicality. But we’ve shown that it’s possible to do real chemistry with real gas mixtures—even when water and unreacted CO₂ are present.”
Not Quite Ready for Prime Time—Yet
While the proof-of-concept is a major scientific step, the researchers acknowledge that there’s still work to be done. The plastic formed in their experiment doesn’t yet match the quality or molecular weight of conventional polyketones produced from fossil fuels. But the path forward is clearer now.
“We’re not saying this is ready for industrial scale-up tomorrow,” says Agapie. “But we’ve demonstrated a working system. And just as importantly, we’ve shown it’s possible to integrate two very different chemical processes into one loop that’s powered by clean electricity.”
The team also emphasized that any future real-world application would need to be powered by renewable energy sources—like solar or wind—if the process is to remain genuinely sustainable.
The Future of Carbon Utilization
This breakthrough isn’t just about making plastic. It’s about changing how we think of carbon dioxide—from waste to wealth.
In a world racing to decarbonize, technologies like this offer a new approach: not just cutting emissions, but actively turning them into building materials. The implications stretch beyond just plastic. Systems like this could be adapted to produce fuels, fertilizers, or pharmaceutical precursors—all from CO₂ and electricity.
The key challenge, as always, will be scaling. Can this be made cost-effective, durable, and energy-efficient enough for real-world deployment? That’s the next frontier. But the Caltech team’s work lights a clear path toward it.
“Every great technological shift starts with a new way of seeing the familiar,” says Agapie. “We’ve long thought of carbon dioxide as a problem. But what if it’s part of the solution?”
As the climate clock ticks, turning carbon into plastic may become one more tool in humanity’s fight to undo the damage already done—proof that even our dirtiest waste can be reborn as something useful, clean, and enduring.
Reference: Maxim Zhelyabovskiy et al, Plastic from CO2, Water, and Electricity: Tandem Electrochemical CO2 Reduction and Thermochemical Ethylene‐CO Copolymerization, Angewandte Chemie International Edition (2025). DOI: 10.1002/anie.202503003