Imagine a roaring bonfire on a cold winter night. The warmth radiates from the burning wood, drawing people closer. Now picture a cool pack used on a sprained ankle, suddenly chilling upon activation. Both of these everyday occurrences are manifestations of the same fundamental chemical principle: the exchange of energy in chemical reactions.
This article will take you deep into the fiery heart and icy edges of chemical change—exploring the fascinating world of endothermic and exothermic reactions. These reactions are more than just textbook topics; they shape the world around us, from the biological processes within our cells to the stars burning in distant galaxies.
In this comprehensive journey, we will explore what these reactions are, how they work, how energy moves during chemical changes, and how this knowledge fuels industries, medicine, cooking, engineering, and even the life force in living organisms.
Welcome to the elegant dance of heat and matter, where atoms don’t just react—they ignite or chill the world around them.
The Basics of Chemical Reactions
Before diving into the heat of the moment, we need to understand what a chemical reaction actually is.
A chemical reaction is a process where one or more substances (called reactants) are transformed into different substances (called products). During this transformation, bonds between atoms are broken and new ones are formed.
Breaking bonds requires energy, while forming bonds releases energy. The overall energy change determines whether a reaction is endothermic or exothermic.
In short:
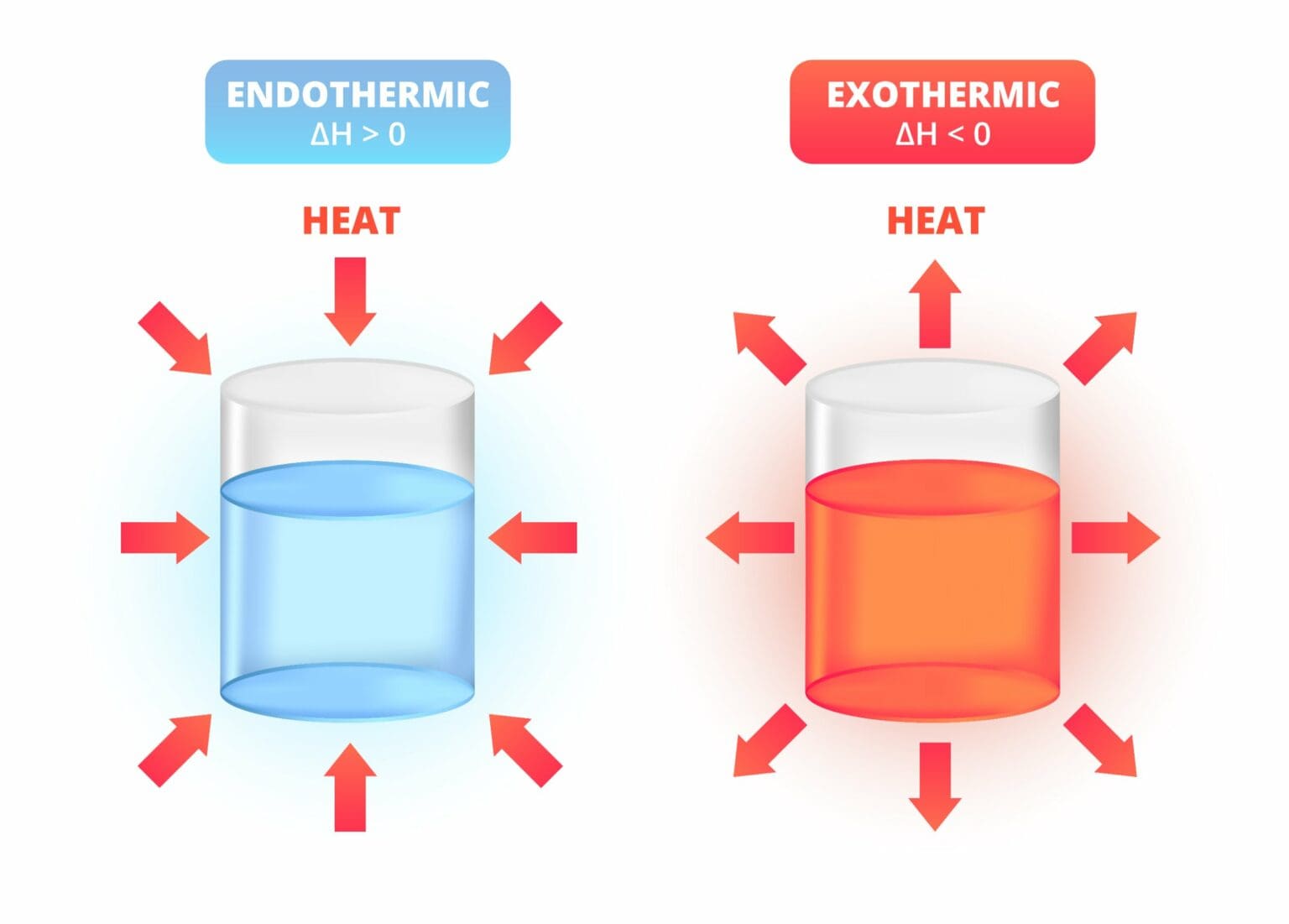
- Endothermic reaction: Absorbs heat (feels cold).
- Exothermic reaction: Releases heat (feels hot).
These energy exchanges are part of a broader field known as thermochemistry, which studies the heat involved in chemical and physical processes.
Let’s dive deeper into each type.
Endothermic Reactions: Pulling Heat In
The word “endothermic” comes from the Greek roots: “endo” meaning “within” and “thermic” meaning “heat.” So, an endothermic reaction is one that absorbs heat from its surroundings.
This results in a drop in temperature around the reaction site. You’ve probably felt this if you’ve ever touched an instant cold pack—when activated, it feels chilly because the reaction inside it is absorbing heat from your skin.
How It Works
At the molecular level, endothermic reactions occur when:
- The energy required to break bonds in the reactants is greater than the energy released when new bonds are formed in the products.
- The excess energy needed must come from the environment, usually in the form of heat.
This absorbed energy is not lost; it becomes part of the chemical potential of the products.
Common Examples
1. Photosynthesis
Arguably the most famous endothermic process is photosynthesis, the miracle at the heart of life on Earth.
Plants absorb sunlight (a form of energy) to convert carbon dioxide and water into glucose and oxygen. 6CO2+6H2O+light energy→C6H12O6+6O26CO_2 + 6H_2O + \text{light energy} \rightarrow C_6H_{12}O_6 + 6O_2
This reaction absorbs energy from sunlight, storing it in the glucose molecules that animals (including us) later consume.
2. Evaporation of Water
When water evaporates, it absorbs heat from the environment to break the bonds holding its molecules together as a liquid. This is why sweating cools us down—evaporation is endothermic.
3. Thermal Decomposition
In reactions where a compound breaks down into two or more simpler substances using heat, such as the decomposition of calcium carbonate into calcium oxide and carbon dioxide, heat must be absorbed. CaCO3→ΔCaO+CO2CaCO_3 \xrightarrow{\Delta} CaO + CO_2
Applications
- Instant cold packs for sports injuries.
- Cooking: Melting, boiling, and baking typically require heat input.
- Photosynthesis-based biofuels: Capturing and storing solar energy for later use.
- Industrial processes like metal refining, where ores are broken down at high temperatures.
How We Measure Energy In
Scientists measure energy absorbed using enthalpy change (ΔH). For endothermic reactions, ΔH is positive, indicating heat is absorbed.
Exothermic Reactions: Releasing the Fire
“Exo” means “outside”—so exothermic reactions release heat into their surroundings. These are the reactions that feel hot.
From the combustion in your car engine to the warmth from your laptop battery to the detonation of explosives, exothermic reactions power much of modern life.
How It Works
In exothermic reactions:
- The energy released when new bonds form in the products is greater than the energy needed to break the bonds in the reactants.
- This excess energy is released, often as heat, light, or even sound.
The surrounding environment gets warmer, and sometimes the results are quite explosive.
Common Examples
1. Combustion
Burning fuels like wood, coal, gasoline, or natural gas are all exothermic reactions.
For instance, the combustion of methane: CH4+2O2→CO2+2H2O+heatCH_4 + 2O_2 \rightarrow CO_2 + 2H_2O + \text{heat}
The energy released powers cars, heats homes, and even launches rockets.
2. Respiration
Just as photosynthesis stores energy, cellular respiration releases it. C6H12O6+6O2→6CO2+6H2O+energyC_6H_{12}O_6 + 6O_2 \rightarrow 6CO_2 + 6H_2O + \text{energy}
This is how your body turns food into usable energy—via an exothermic process inside your cells.
3. Neutralization Reactions
Mixing an acid and a base (like vinegar and baking soda) produces heat. These acid-base reactions are often exothermic.
4. Rusting of Iron
Even slow reactions like oxidation of metals release energy over time. Rusting iron, though less dramatic, is exothermic.
Applications
- Heating: Combustion in fireplaces, stoves, engines.
- Power generation: Burning fossil fuels in power plants.
- Explosives: Rapid exothermic reactions release massive energy.
- Batteries: Chemical energy released during discharge is exothermic.
- Self-heating cans: Use exothermic reactions to warm food.
Measuring the Heat
In exothermic reactions, ΔH is negative—indicating that energy is being lost from the system to the environment.
The Role of Activation Energy
Whether a reaction is exothermic or endothermic, it usually needs a kickstart: a minimum amount of energy to begin. This is called activation energy.
Think of it as pushing a boulder over a hill: the boulder won’t roll down (release energy) until you get it up to the peak (activation energy).
- Endothermic reactions often require more activation energy.
- Exothermic reactions can be self-sustaining once they start.
This is why wood doesn’t spontaneously combust—it needs the heat of a match to get going.
Reaction Profiles: Seeing the Energy
Chemists often visualize energy changes using reaction profile diagrams.
In these diagrams:
- The vertical axis shows energy.
- The horizontal axis shows progress of reaction.
- For exothermic reactions, the products are at a lower energy level than reactants.
- For endothermic reactions, the products are at a higher energy level.
The peak between reactants and products represents activation energy.
Reversible Reactions and Equilibrium
Some reactions are reversible—meaning they can go forward and backward.
Example: NH4Cl⇌NH3+HClNH_4Cl \rightleftharpoons NH_3 + HCl
In such cases:
- The forward reaction might be endothermic.
- The reverse reaction will be exothermic.
Understanding this balance is crucial in chemical engineering, especially in processes like the Haber process for making ammonia.
Biological Relevance: Life’s Balancing Act
Living organisms use a combination of endothermic and exothermic reactions to survive.
- Exothermic reactions in the body (like ATP hydrolysis) power muscles, nerves, and cellular machinery.
- Endothermic reactions are used for synthesis—building DNA, proteins, and new cells.
The balance is tightly regulated. If too much energy is released too quickly (like in fever or inflammation), damage occurs. If energy absorption is too great (as in hypothermia), systems shut down.
Industrial Applications
Chemical industries thrive on controlling these energy changes.
Exothermic Processes:
- Cement and concrete curing (releases heat).
- Steelmaking: Exothermic oxidation of impurities.
- Power plants: Controlled combustion to produce electricity.
Endothermic Processes:
- Electrolysis: Breaking water into hydrogen and oxygen using electrical energy.
- Smelting: Requiring heat to extract pure metals.
Managing energy flow is critical for safety, efficiency, and environmental impact.
Everyday Chemistry: Reactions All Around Us
- Cooking is full of chemical changes. Boiling water is endothermic, while caramelizing sugar is exothermic.
- Baking soda and vinegar volcanoes produce heat.
- Glow sticks are a great example of chemiluminescence, often exothermic.
Even emotional warmth—like the heat from someone’s touch—can be understood through chemical principles. Our bodies are walking chemistry labs, with a perfect blend of reactions happening every moment.
Experimental Fun: Feeling the Heat and the Chill
Science experiments can make these concepts tangible.
- Mixing baking soda and citric acid with water creates an endothermic reaction—feels cold.
- Mixing calcium chloride with water is exothermic—feels warm.
These simple reactions are safe and visually demonstrate the principles of thermochemistry.
Conclusion: Two Sides of the Same Coin
Endothermic and exothermic reactions are the yin and yang of the chemical world. They reflect the fundamental truth that energy cannot be created or destroyed—only transformed.
In every flame, in every breath, in every cell and star, these reactions shape existence. They are as intimate as the digestion of your breakfast and as grand as a supernova.
By understanding them, we don’t just understand chemistry—we understand life, technology, energy, and the cosmos.
Let this be the spark that ignites your passion for chemistry.