Every second of every day, trillions of chemical reactions unfold around us and within us. From the combustion of fuel in engines to the metabolism in your cells, from rust forming on a bicycle chain to the baking of a loaf of bread—chemistry is the beating heart of the physical world. And behind each of these reactions lies a hidden player, an unsung force that determines whether a chemical change will take place at all.
This mysterious force is called activation energy—a crucial concept in chemistry and physics, and one of the key ideas that links energy, motion, and transformation. Activation energy is the threshold a reaction must overcome to begin. It’s the “starter’s pistol” of the molecular world. Without it, nothing reacts. Nothing changes.
In this deep dive, we’ll explore what activation energy really is, how it works, why it matters in everyday life, and how it shapes everything from biology to engineering to the edge of human understanding. Prepare to journey into the very moment before change happens—the moment of potential, the spark before the flame.
A Thought Experiment: Striking a Match
Imagine holding a matchstick in your hand. The chemicals on the match head are perfectly capable of burning. Oxygen is present in the air. The wood of the matchstick is dry and ready to be consumed by flame. So why doesn’t it burst into fire the moment you take it out of the box?
The answer is simple: the reactants (the match head and the oxygen) do not yet have enough energy to start the reaction. But the moment you strike the match, friction generates heat. That heat gives the atoms in the match head enough energy to move faster, collide harder, and rearrange their bonds in a rapid oxidation reaction—commonly known as fire.
That extra push, that initial burst of energy that gets the chemical reaction going, is what we call activation energy.
Defining Activation Energy
In scientific terms, activation energy (Ea) is the minimum amount of energy required to convert reactants into products during a chemical reaction. It’s the energy barrier that must be overcome for a reaction to proceed.
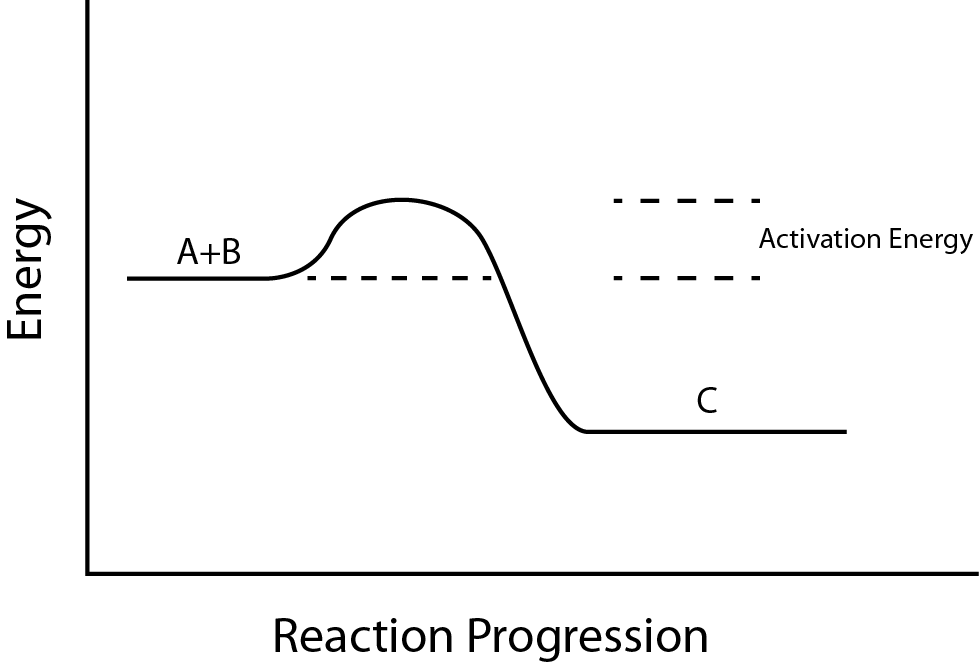
Think of it as a hill between two valleys. In the first valley, you have your starting substances—your reactants. On the other side lies the second valley—your products. To get from one to the other, the system must climb the hill: the activation energy.
This concept was first introduced by Swedish scientist Svante Arrhenius in 1889, and it became a foundational piece of what’s known as transition state theory—a branch of chemistry that studies how chemical reactions actually occur at the molecular level.
The Energy Landscape of a Reaction
To understand activation energy better, imagine a graph where the vertical axis represents the energy of the system, and the horizontal axis shows the progress of the reaction.
At the beginning of the reaction, the system is at a certain energy level (the reactants). To start the reaction, energy must be added to raise the system to the transition state—the high-energy point where the old bonds are partially broken, and new bonds are partially formed. This is the most unstable, fleeting moment in the reaction—like the crest of a rollercoaster just before it plunges downward.
Once the system crosses this peak, the reaction proceeds “downhill,” releasing energy and forming products. The difference between the starting energy and the peak is the activation energy.
In exothermic reactions, the products have lower energy than the reactants, and energy is released (as heat, light, or sound). In endothermic reactions, the products end up at a higher energy state, and energy must be absorbed for the reaction to occur.
Microscopic View: Collisions and Transition States
Let’s zoom in even further, into the molecular scale.
Chemical reactions happen when molecules collide with one another in the right way—with the right amount of energy and in the right orientation. Not all collisions lead to reactions. In fact, most collisions between molecules are like two cars bumping in a parking lot—no lasting damage, no big change. But occasionally, the impact is just right. Bonds stretch, snap, and rearrange.
This is where the concept of effective collisions comes into play. For a reaction to occur:
- Molecules must collide.
- The collision must involve enough energy (activation energy).
- The molecules must be oriented correctly.
If all three conditions are met, the reactants pass through the transition state, and products are formed.
The Arrhenius Equation: Quantifying Activation Energy
To mathematically understand how activation energy affects reaction rate, we turn to the Arrhenius equation:
k = Ae^(-Ea/RT)
Where:
- k is the rate constant of the reaction,
- A is the frequency factor (related to how often molecules collide in the right orientation),
- Ea is the activation energy,
- R is the gas constant,
- T is the absolute temperature (in Kelvin).
This equation tells us something profound: even a small increase in temperature can lead to a large increase in the reaction rate, because more molecules have the energy to overcome the activation barrier.
It also shows why reactions with high activation energy are slow—unless something is done to speed them up.
Catalysts: Lowering the Barrier
One of the most fascinating aspects of activation energy is how it can be manipulated—specifically, how it can be lowered. This is the job of catalysts.
A catalyst is a substance that speeds up a chemical reaction by providing an alternative reaction pathway with a lower activation energy. It does not get consumed in the reaction—it merely helps guide the reactants to the products more efficiently.
In our match example, if we coated the match head with a chemical catalyst, it might ignite with just a whisper of friction—or even spontaneously under the right conditions.
In biological systems, catalysts are essential. These biological catalysts are known as enzymes, and they are the reason life can exist at all. Without enzymes, most of the chemical reactions your body needs would occur too slowly to support life. Your digestion, your breathing, even your thoughts depend on enzymes lowering the activation energy of biochemical reactions.
Everyday Examples of Activation Energy
Activation energy is not just a classroom concept—it’s at work in every corner of daily life.
When you cook food, you are providing heat to overcome the activation energy of various chemical reactions—like the Maillard reaction, which gives browned food its delicious flavor. When you charge your phone, activation energy is involved in the electron transfers in the battery. When you light a candle, set off fireworks, or even digest food, activation energy is there, waiting to be surpassed.
One of the clearest examples is in explosives. These substances are highly energetic but also require an initial trigger to set them off—such as a spark, impact, or heat. That trigger provides just enough energy to surpass the activation threshold, after which the reaction proceeds explosively, releasing massive amounts of energy.
On the flip side, fire-retardant materials are designed to raise the activation energy of combustion reactions, making it harder for fire to start.
The Biology of Activation Energy
In the microscopic machinery of life, activation energy is everywhere.
Cells are chemical factories. Thousands of reactions occur every second—breaking down glucose for energy, synthesizing proteins, copying DNA. All of these reactions require activation energy. But because the internal temperature of a living organism is relatively low, cells need enzymes to catalyze these reactions efficiently.
Each enzyme is specific to a reaction. It works by binding to the reactants (called substrates), distorting their shape and reducing the amount of energy needed to reach the transition state.
The result? Reactions that would take days or years in a test tube happen in milliseconds in your body.
Temperature, pH, and other factors affect the efficiency of enzymes—and thus the activation energy they provide. That’s why a high fever can be dangerous: too much heat can denature enzymes, stopping critical reactions in their tracks.
Activation Energy and Industrial Chemistry
In the world of industry, control over activation energy is a matter of efficiency, cost, and safety.
Take the Haber-Bosch process, which synthesizes ammonia from nitrogen and hydrogen. This reaction is essential for fertilizer production and supports the global food supply. But nitrogen molecules are incredibly stable, with a very high activation energy.
To make this reaction viable, engineers use high pressures, high temperatures, and an iron-based catalyst to lower the activation energy and speed up the reaction.
In petroleum refining, pharmaceutical synthesis, and polymer production, chemists constantly tweak reaction conditions to optimize activation energy. Too high, and the process is slow or energy-wasting. Too low, and you risk uncontrolled reactions, explosions, or inefficiencies.
Activation Energy in the Natural World
Nature itself is a master chemist. Lightning provides activation energy for the formation of nitric oxide in the atmosphere. Volcanic heat triggers chemical changes that release minerals. Forest fires, while devastating, release nutrients back into the soil—after enough activation energy (heat) breaks down plant material.
Even evolution has a metaphorical version of activation energy. Change doesn’t happen until there’s enough pressure—environmental, genetic, or otherwise—to force an organism or population over a threshold of adaptation.
In this way, activation energy becomes a broader concept: the idea that transformation requires effort, a push, a spark.
The Philosophical Angle: Thresholds of Change
Activation energy doesn’t just belong to chemistry. It’s a metaphor for life.
How many times have you sat in front of a project, needing just a little nudge to begin? That moment—the mental inertia, the inner friction—is psychological activation energy. The smallest action—a phone call, a sentence, a breath—can lower that barrier and get you started.
In economics, social change, innovation, even politics—no shift happens without crossing a threshold. Something must tip the balance. And once it does, the change is rapid, transformative, irreversible.
This makes activation energy not just a scientific term, but a symbol of all transformation.
Conclusion: The Quiet Power Behind Every Reaction
Activation energy is invisible, yet essential. It is the cost of change, the toll gate on the road to reaction. Whether we are igniting a fire, digesting a meal, building a bridge, or contemplating the beginning of the universe, activation energy is there—guiding the transition from what is to what could be.
It explains why some reactions are slow and some are explosive. It teaches us how to control, harness, and accelerate change. It bridges physics and chemistry, theory and practice, the molecular and the monumental.
And in its quiet way, it reminds us that nothing changes—nothing moves—without that first spark.