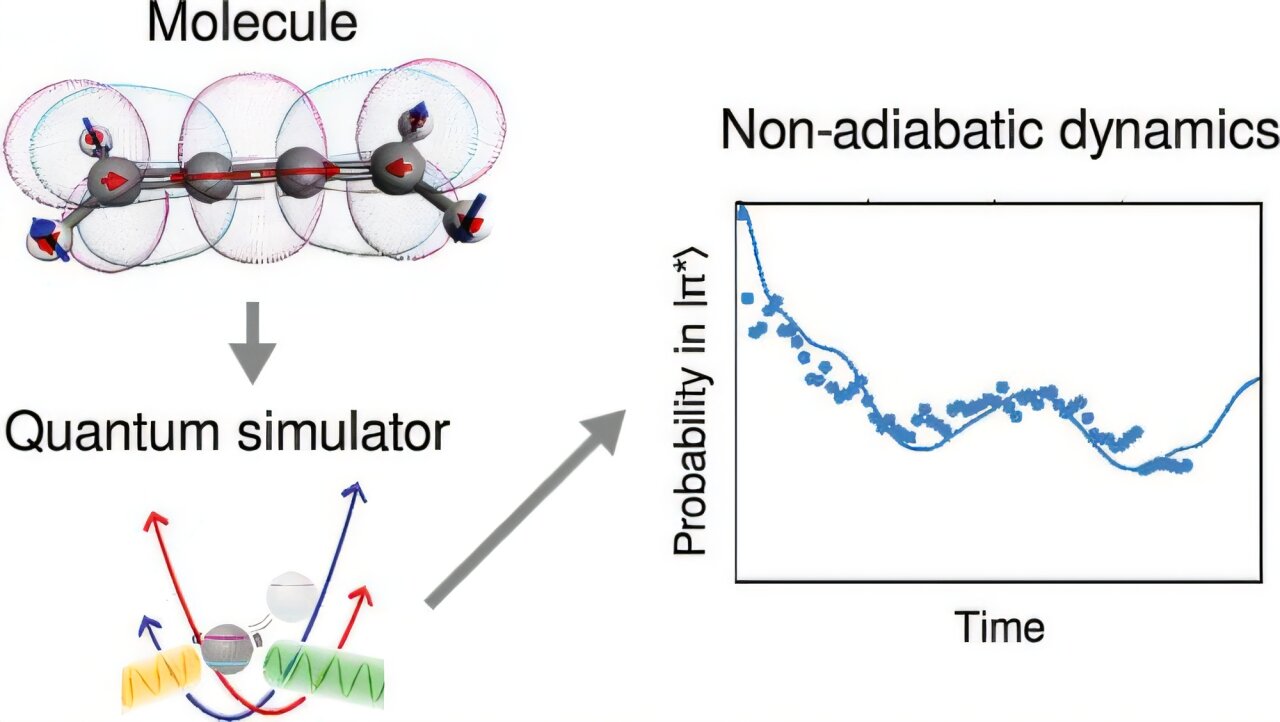
In the heart of the University of Sydney’s Nanoscience Hub, something extraordinary has happened—an innovation that could forever change how we understand the chemistry of life, medicine, and matter. For the first time, researchers have used a quantum computer to simulate the dynamic behavior of real molecules—not just their static states, but how they dance, twist, vibrate, and transform when struck by light.
Led by quantum chemist Professor Ivan Kassal and Physics Horizon Fellow Dr. Tingrei Tan, this work doesn’t just edge forward our understanding of quantum technology; it catapults it into an entirely new realm. What they’ve done isn’t just important—it’s revolutionary.
This achievement, recently published in the Journal of the American Chemical Society, marks a defining moment in quantum simulation, where science fiction edges ever closer to reality.
Beyond the Still Life of Atoms
Until now, quantum computers—even in their advanced forms—have been able to calculate the energies and stable configurations of molecules. It’s as if we could take a still photograph of a molecule frozen in time, measuring its shape, its bonds, and how much energy it holds. Useful, yes—but it’s a far cry from understanding how that molecule behaves under real-world conditions.
Because chemistry—real chemistry—is alive. It’s dynamic. It happens in time.
From how chlorophyll harvests sunlight to how UV light damages DNA, the most important chemical processes are fast, furious, and light-triggered. They happen in femtoseconds—a millionth of a billionth of a second—and they have long been impossible to fully model using even the most powerful classical computers.
But now, for the first time, we can watch those reactions unfold with quantum precision. This breakthrough isn’t a tweak in technique. It’s the difference between studying a photograph and watching a movie—frame by frame, second by second—of nature’s most complex interactions.
The Mountain Hiker Metaphor
Professor Kassal uses a vivid analogy to explain the conceptual leap. “It is one thing to understand your starting point, your end point, and how high you’ll need to climb,” he says. “But this doesn’t help you understand the path you will take.”
Classical simulations have, until now, mapped only the starting and ending points of chemical journeys—energy levels, reaction products. What they lacked was the journey itself: the real-time motion of atoms, the push and pull of electron clouds, the choreography of energy transfer.
The new approach simulates those dynamic paths, offering a moving picture of how light energizes molecules, how electrons leap to new orbits, and how atoms shift in response.
It’s like tracking a hiker on a twisting trail through fog-shrouded mountains—not just seeing where they start and end, but witnessing every footfall, every climb, every stumble, and every view along the way.
From Abstraction to Reality: Simulating Actual Molecules
What sets this work apart from prior efforts in quantum dynamics is the transition from abstract models to real molecules. This isn’t a toy model or a proof-of-concept involving fictitious atoms. The molecules under study—allene (C₃H₄) and butatriene (C₄H₄N₂H₄)—are chemically real, with genuine applications and relevance in fields like organic synthesis and photochemistry.
And these molecules weren’t merely being studied at rest. The researchers simulated what happens when light hits them—how the molecules respond electronically and vibrationally, how they wobble, twist, and shift with each quantum of energy absorbed. This level of simulation, especially in real molecules, has previously been beyond the reach of both classical computers and most quantum machines.
Slowing Down Time to Understand the Fastest Processes in Nature
To simulate events that occur in femtoseconds—millionths of a billionth of a second—the team used a clever trick: time dilation. They slowed down the chemical dynamics by a factor of 100 billion, allowing them to unfold within the manageable span of milliseconds inside their quantum simulator.
It’s akin to watching a hummingbird’s wings, which beat 70 times per second, slowed down to see each feather flutter. Or better yet, like filming lightning and playing it back in slow motion to see how it branches, curves, and arcs before striking.
This approach doesn’t just make the process visible—it allows scientists to measure, manipulate, and understand what was previously too fast to follow.
Why Quantum, and Why Now?
Quantum computers are uniquely suited to this task because molecules themselves are quantum systems. Every bond, every electron interaction, is governed by the strange laws of quantum mechanics. Classical computers, no matter how powerful, must resort to shortcuts and approximations that fall apart as complexity rises.
The more atoms involved, the more exponential the computational cost becomes. Simulating a simple molecule might take hours. A moderately complex one might take days. A realistic, drug-sized molecule reacting with light? That could take years—or simply be impossible to compute using classical means.
But quantum computers don’t face that same roadblock. They scale naturally with quantum systems. As a result, they can theoretically simulate chemical phenomena with unmatched accuracy and efficiency—and that’s exactly what this breakthrough hints at.
The Minimalist Masterstroke: One Ion, Millions of Possibilities
Here’s where things get even more extraordinary: the simulation was carried out using just one trapped ion.
In traditional quantum computing, simulating something this complex would typically demand 11 perfect qubits and upwards of 300,000 high-fidelity entangling gates—a resource burden that remains out of reach for even the best quantum machines today.
But the University of Sydney team took a different path. They used an analog quantum simulation approach, encoding the information into a single ion using a carefully constructed, resource-light scheme. This meant they could simulate real-time chemical dynamics a million times more efficiently than digital quantum methods.
It’s quantum minimalism at its finest: less hardware, more insight.
A Future Rewritten by Quantum Chemistry
The implications of this achievement are staggering.
Light-driven chemical dynamics are at the heart of so many biological, medical, and energy-related processes. Think photosynthesis, the way plants turn sunlight into energy. Think DNA damage from ultraviolet rays, which can trigger mutations and cancer. Think photodynamic therapy, a cutting-edge cancer treatment where light activates a drug to kill targeted cells. Think sunscreen, solar cells, even next-generation semiconductors.
In all these areas, our understanding remains limited because we cannot see the ultrafast processes at play—not clearly, not fully.
But now we can simulate them, with fidelity and precision. And that could unlock everything from better medicines to more efficient energy systems, from customized light-sensitive materials to faster paths in drug discovery.
Dr. Tan puts it simply: “In all these cases, the ultrafast photo-induced dynamics are poorly understood. Having accurate simulation tools will accelerate the discovery of new materials, drugs, or other photoactive molecules.”
A Turning Point in Scientific Discovery
This isn’t just a milestone in chemistry or quantum computing—it’s a turning point in how we study nature itself. For centuries, chemistry has advanced through trial and error, through reaction and observation. Then came computational chemistry, where models helped guide discoveries. But quantum simulation promises a future where we can experiment inside the machine, trying out reactions in virtual, quantum-real environments before they’re ever attempted in the lab.
Imagine designing a new solar cell material and testing its performance under sunlight—without ever synthesizing it. Or screening cancer drugs based on how their excited electrons interact with DNA—before moving to animal trials. Or tailoring a material’s optical response molecule by molecule—before lifting a pipette.
The future of science may very well be one where quantum simulations guide our every step—from hypothesis to experiment to solution.
The Road Ahead
While this is a monumental achievement, it’s still an early step. The method, though efficient, was applied to a small set of molecules. Scaling it up to larger systems and integrating it into broader workflows will require continued innovation—both in quantum hardware and in the theories that support it.
But the direction is clear. What was once theoretical is now experimental. What was once impossible is now achievable.
The University of Sydney team hasn’t just opened a door—they’ve kicked down a wall between physics and chemistry, between theory and application, between what we could imagine and what we can now simulate.
And as this quantum journey continues, we may look back on this moment as the dawn of a new era in science—when we stopped guessing at how molecules moved and started watching them in action.
Reference: Tomas Navickas et al, Experimental Quantum Simulation of Chemical Dynamics, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c03336