In an extraordinary collaboration, researchers from UC Santa Barbara, the University of California San Francisco (UCSF), and the University of Pittsburgh have developed a revolutionary method to design enzymes from scratch—ushering in a new era in chemistry that could reshape industries ranging from pharmaceuticals to green energy. The study, recently published in Science, unveils a powerful workflow for creating entirely novel enzymes, also known as de novo proteins, capable of performing reactions previously thought to be the exclusive domain of natural biological systems.
This breakthrough, driven by the synergistic efforts of the Yang Lab (UCSB), the DeGrado Lab (UCSF), and the Liu Lab (University of Pittsburgh), marks a turning point in our ability to design and deploy bespoke catalysts tailored for high efficiency, selectivity, and resilience in non-natural environments.
Why Enzymes Matter—and Why We Need New Ones
Enzymes are the molecular machines that make life possible. These naturally occurring protein catalysts control the speed and specificity of countless biochemical reactions in all living organisms. In the world of chemistry, they are often referred to as nature’s privileged catalysts due to their unparalleled selectivity and efficiency. Yet for all their power, natural enzymes come with limitations—they tend to be sensitive to changes in temperature, pH, or solvents, and are often restricted to working with specific substrates in narrow biological contexts.
This is where the promise of de novo enzyme design shines. By building new proteins from the ground up using carefully chosen amino acid sequences, researchers can not only bypass the limitations of natural enzymes but also expand their catalytic capabilities into uncharted chemical territory.
As UCSB chemist Yang Yang, one of the senior authors of the paper, puts it: “If people could design very efficient enzymes from scratch, you could solve many important problems.” From cleaner chemical manufacturing to better drug synthesis and even novel materials creation, the applications are as vast as they are exciting.
Designing the Future: Building from First Principles
Traditional protein engineering often relies on tweaking existing enzymes—tuning their amino acid sequences to improve performance. In contrast, de novo design starts with a blank slate. Instead of modifying nature’s blueprints, scientists write their own, using amino acids like a molecular alphabet to compose proteins with predetermined shapes and catalytic functions.
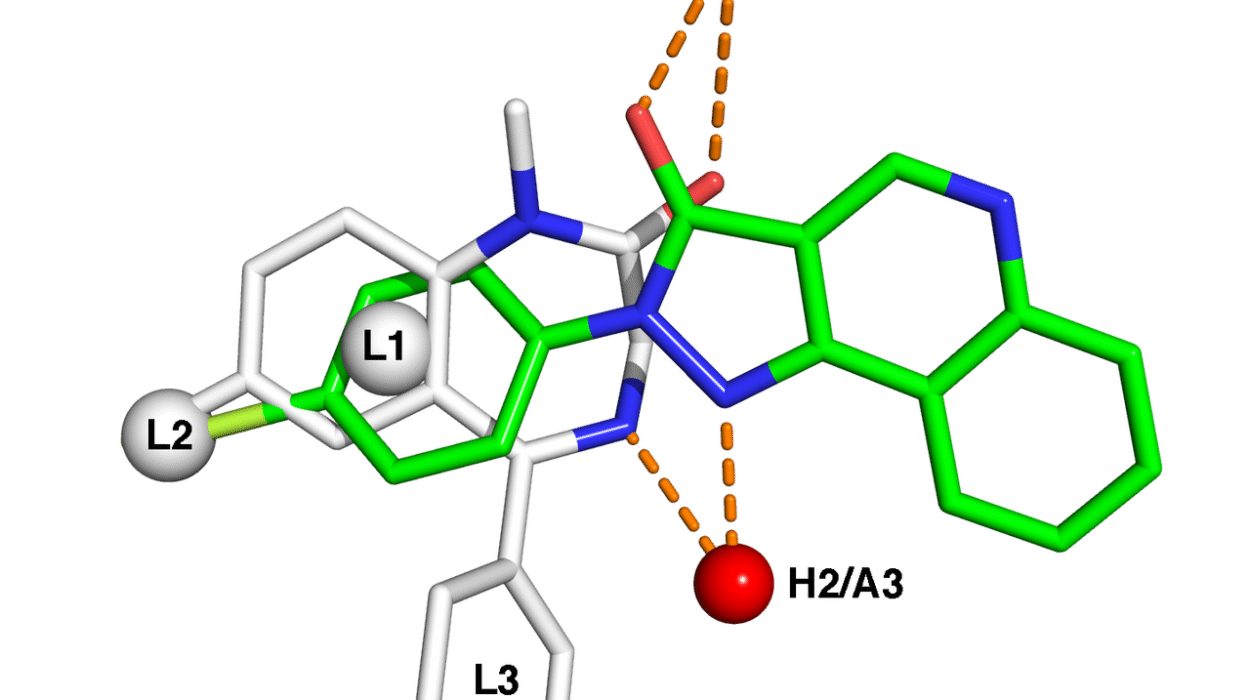
In this particular study, the research team focused on a class of synthetic proteins known as helical bundles. These compact, stable structures serve as a scaffold for designing active sites—regions within the protein where catalysis occurs. One of the advantages of these synthetic proteins is their resilience: they can tolerate higher temperatures, thrive in organic solvents, and accommodate cofactors—molecules that aid enzyme function—that are foreign to nature.
These customized proteins were not only small and efficient but also capable of carrying out key chemical transformations, such as forming carbon-carbon and carbon-silicon bonds. These types of reactions are vital in organic synthesis, particularly in pharmaceuticals and advanced materials, yet natural enzymes rarely catalyze them efficiently.
The AI–Human Hybrid Approach
To design the first generation of enzyme candidates, the team turned to advanced artificial intelligence models. These AI systems predicted which amino acid sequences would fold into the desired protein structures and possess catalytic activity. While the early results were promising, the enzymes exhibited only moderate efficiency and selectivity.
Upon further investigation using X-ray crystallography—a technique that reveals the three-dimensional arrangement of atoms in a protein—the researchers identified a structural flaw: a loop within the protein that failed to adopt the intended helical structure, weakening the active site.
This is where human ingenuity stepped back in. Leveraging their own algorithm and chemical intuition, the researchers launched a second design round, this time incorporating a loop-searching strategy to improve structural stability. The result? Four out of ten newly designed proteins displayed remarkable catalytic activity and stereoselectivity—key qualities for industrial and pharmaceutical applications.
As Yang noted, “Although AI-based protein design methods are very useful, to have very good catalysts we still have to use our in-house algorithm and our chemical intuition to get everything done the right way.”
The Green Chemistry Advantage
One of the most compelling features of de novo enzyme design is its compatibility with sustainable chemistry. Traditional synthetic catalysts often require toxic solvents, heavy metals, or extreme conditions. In contrast, engineered enzymes can perform reactions in water—the greenest and most benign solvent available—and often do so at ambient temperatures and pressures.
This environmentally friendly approach not only reduces waste and energy consumption but also opens the door to greener manufacturing processes in sectors such as agriculture, fine chemicals, and even environmental remediation.
“If you really understand the design principles,” said Yang, “then you can build a protein catalyst to use whatever cofactors you would like, and to achieve challenging transformations in water.” That level of customization, combined with the environmental benefits, makes de novo enzymes a compelling alternative to traditional chemical methods.
Expanding the Frontiers of Molecular Possibility
The implications of this breakthrough stretch far beyond the lab. By proving that synthetic helical bundle proteins can be transformed into highly active and selective enzymes, this research lays the groundwork for the routine design of tailored biocatalysts. These designer enzymes can potentially be crafted to perform specific transformations on demand, vastly broadening the scope of what is chemically possible.
Moreover, this new design framework also allows researchers to explore entirely new catalytic mechanisms—those not found in nature. This aspect of de novo design is particularly exciting because it means scientists can invent enzymatic functions that evolution never explored, unlocking new reactions and synthetic pathways for complex molecules.
In the near future, the team plans to push the boundaries even further. The next steps include mimicking complex functions of natural enzymes using smaller and simpler synthetic proteins, as well as designing enzymes that operate through unprecedented mechanisms. These goals could not only deepen our understanding of enzymology and protein chemistry but also bring about powerful new tools for drug development, synthetic biology, and material science.
A New Language of Life
At its core, this research exemplifies a profound shift in how we interact with biology. Rather than merely reading the book of life, we’re beginning to rewrite it—creating new molecular tools that didn’t exist in nature but are nonetheless grounded in biological principles.
What once required the slow grind of evolutionary pressure can now be achieved in a laboratory, guided by human creativity, machine learning, and a deep understanding of molecular structure. The fusion of AI-driven design and chemical expertise enables an era in which we no longer have to wait for nature to deliver the right enzyme—we can simply build it ourselves.
In many ways, this is the molecular equivalent of going from using hand tools to designing custom robotic arms. The precision, efficiency, and versatility of de novo enzymes make them the chemical powerhouses of the future.
Looking Ahead: Engineering Tomorrow’s Chemistry
The success of this project has not only proven the power of de novo design but has also set the stage for widespread adoption of this approach. In the coming years, we can expect to see an explosion of new enzymes tailored for specific industrial processes, therapeutic interventions, and even environmental applications such as waste breakdown or carbon capture.
The road ahead is one of thrilling possibility. With continued innovation, deeper integration of AI and quantum computing, and increasing collaboration across scientific disciplines, de novo protein design will likely become a cornerstone of 21st-century chemistry.
As Yang concludes, “This is just the beginning. If we can master the art of enzyme design, we can truly reimagine what’s possible in science, technology, and sustainability.”
Reference: Kaipeng Hou et al, De novo design of porphyrin-containing proteins as efficient and stereoselective catalysts, Science (2025). DOI: 10.1126/science.adt7268. www.science.org/doi/10.1126/science.adt7268