Deep within the pressure chambers of cutting-edge laboratories, scientists have long been chasing a dream: the creation of superhydrides—materials capable of storing immense amounts of hydrogen. These compounds promise nothing short of technological revolution. With their potential to act as next-generation superconductors and ultra-efficient hydrogen storage materials, superhydrides could reshape transportation, quantum computing, and the broader clean energy landscape.
But there’s a catch—an enormous one. Superhydrides are incredibly difficult to make. Forming them requires pressures so extreme they mimic the heart of gas giant planets like Jupiter—pressures measured in tens of gigapascals, where atoms behave in profoundly unfamiliar ways. Even in the most advanced high-pressure experiments, these reactions behave like mischievous riddles: elusive, unpredictable, and agonizingly sensitive to minor changes.
For more than a decade, the synthesis of just one such compound—calcium superhydride (CaH₆)—was a holy grail that refused to be claimed. Theoretical predictions suggested the material could exist, and its superconducting potential made it an irresistible target. But experimental attempts yielded nothing conclusive—until recently.
Now, a team of international researchers has changed the game entirely. By using machine learning to model the ultra-high-pressure reaction pathways that govern superhydride formation, they’ve unveiled how such compounds emerge, atom by atom. Published in the Proceedings of the National Academy of Sciences on May 29, 2025, their work is not just a triumph of materials science—it’s a landmark moment in the marriage between artificial intelligence and fundamental chemistry.
What Are Superhydrides, and Why Do They Matter?
Hydrides are compounds that incorporate hydrogen atoms into their structure, typically bonded to metals. What makes superhydrides distinct is the sheer density of hydrogen within their molecular framework. For example, CaH₆ contains one calcium atom for every six hydrogen atoms—a ratio that pushes the boundaries of what solid-state chemistry was once believed capable of supporting.
This hydrogen richness gives superhydrides extraordinary properties. Chief among them is the potential for high-temperature superconductivity, a phenomenon where electrical resistance vanishes, and magnetic fields are expelled, allowing for levitating maglev trains or ultra-sensitive quantum devices. In theory, the more hydrogen packed into the structure, the higher the potential temperature at which superconductivity might occur. Some superhydrides have already shown superconducting behavior at temperatures as high as 250 K under pressure—chilly, but far warmer than traditional superconductors that require liquid helium to function.
Moreover, hydrogen’s role as a clean fuel source makes superhydrides promising for hydrogen storage, a cornerstone for a carbon-neutral energy future. They offer dense, stable, and potentially reversible hydrogen storage media—ideal for portable energy systems and grid-scale applications. But tapping into this potential requires understanding and controlling how these compounds are formed.
Why Superhydrides Are So Hard to Make
Making superhydrides is like baking a cake in a collapsing building. The recipe is known in theory, but the required conditions are chaotic and extreme. Temperatures can soar to hundreds of degrees Celsius. More importantly, pressures of 100 GPa or more—nearly a million times atmospheric pressure—must be applied to squeeze atoms into unfamiliar arrangements.
And yet, achieving those conditions is not even the hardest part. The true challenge is knowing what to do once you’re there.
Under such pressures, traditional tools like thermal analysis or spectroscopy falter. Instruments struggle to “see” inside tiny diamond anvil cells where samples are crushed. Reaction pathways—the series of chemical steps that transform starting materials into final products—remain invisible. Scientists have been flying blind, relying on intuition, brute-force experimentation, and occasional lucky guesses.
As Professor Shin-ichi Orimo of the Advanced Institute for Materials Research (WPI-AIMR) puts it: “To give an example of how finicky these reactions are, the synthesis of calcium superhydride, CaH₆, took a decade to achieve from initial structural prediction.”
In the world of materials science, that’s an eternity.
Machine Learning Enters the Pressure Chamber
Enter machine learning—a tool more often associated with self-driving cars or recommendation algorithms than exotic high-pressure chemistry. But here, artificial intelligence has found a new calling.
A research team led by Assistant Professor Ryuhei Sato of the Graduate School of Engineering at the University of Tokyo, in collaboration with Professor Hao Li of WPI-AIMR and Professor Chris Pickard of the University of Cambridge, built a machine learning potential (MLP)—a sophisticated algorithm trained on thousands of data points derived from first-principles quantum mechanical calculations.
This MLP was designed specifically to model the behavior of hydrogen and calcium hydrides under extreme conditions. The model can simulate atomic-scale interactions far faster than traditional quantum simulations while retaining remarkable accuracy.
The researchers fed the model existing information about calcium hydride (CaH₂) and hydrogen, and then let it simulate the behavior of these materials when squeezed under high pressures and heated. The result was like watching a microscopic movie of atoms dancing under duress—a computational time-lapse of the birth of a superhydride.
Unveiling the Reaction Pathway: Melting, Absorption, Solidification
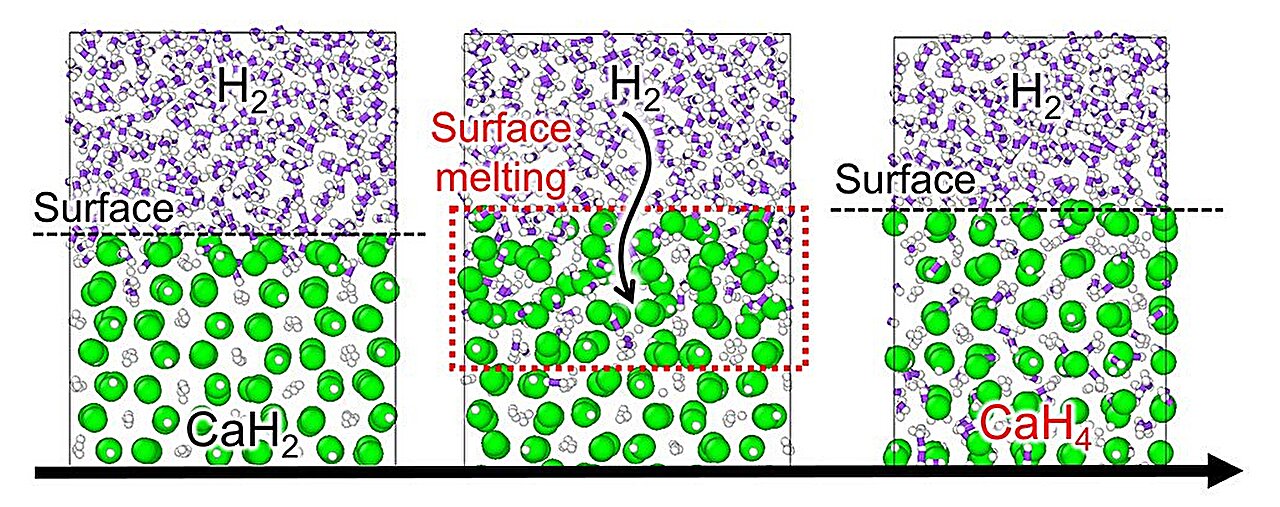
The simulations revealed something unexpected: a three-stage reaction pathway that hadn’t been observed before.
It begins with surface melting. Under sufficient pressure and heat, the outer layer of calcium hydride begins to melt—not the whole material, just the surface. This semi-liquid layer becomes permeable, allowing hydrogen molecules to slip inside. Once inside, the hydrogen dissociates into atoms, diffuses further into the structure, and rearranges the atomic lattice.
Then, as the hydrogen content increases and pressure persists, the material solidifies again, not as CaH₂ but as CaH₄, a precursor to CaH₆. This intermediate phase suggests a stepping-stone pathway—a sequence of metastable structures that eventually lead to the final superhydride.
This process of pressure-induced surface melting and re-solidification, guided by molecular hydrogen interaction, may be a universal mechanism in high-pressure hydrogen chemistry.
According to Professor Orimo, “This study establishes a new frontier for machine learning by demonstrating its ability to predict previously unknown chemical reaction pathways.” It’s not just a neat trick—it’s a paradigm shift.
Why This Matters for the Future of Energy and Superconductivity
Understanding this reaction pathway is like finally finding the missing map to a treasure trove of high-performance materials. With this map in hand, researchers no longer have to rely on years of trial-and-error. They can target specific pressures and temperatures, anticipate reaction intermediates, and design more efficient synthesis protocols.
This advancement paves the way for scalable production of superhydrides, a crucial step toward practical hydrogen storage and room-temperature superconductors—two holy grails of energy and electronics.
For hydrogen energy, being able to safely store and retrieve hydrogen with minimal loss and high density is critical. Superhydrides could offer a cleaner, safer, and more efficient storage option than compressed or liquefied hydrogen tanks.
For superconductivity, controlling the formation of materials like CaH₆ under known conditions may lead to the development of materials that superconduct at higher temperatures and lower pressures. That’s essential for real-world applications, from magnetic levitation (maglev) transport to quantum computers that rely on resistance-free wiring.
And perhaps most importantly, the use of machine learning in this context opens the door to generalizing this approach to other materials. Imagine using similar models to explore carbon-rich structures, novel metallic alloys, or even planetary materials in Earth’s deep interior. The periodic table itself becomes a playground for predictive modeling.
Charting a New Path for Machine Learning in Physical Chemistry
The implications go beyond materials science. This work marks one of the first clear demonstrations that AI can discover entirely new reaction pathways in environments where human intuition and direct experimentation fall short.
While machine learning has made waves in fields like protein folding, speech recognition, and autonomous systems, its application to unexplored chemical regimes under extreme conditions is still in its infancy. This study, by offering a successful blueprint, sets a precedent.
It also demonstrates a broader philosophical point: that the universe operates by rules that are knowable—even when our traditional senses and tools fail. With the right data, structure, and algorithms, machine learning can act as our proxy in realms of pressure, heat, and atomic motion we cannot yet physically reach.
From Laboratory Curiosity to Planetary Potential
As climate change accelerates and demand grows for cleaner, more efficient energy sources, hydrogen is poised to play a central role. But its adoption hinges on breakthroughs in storage, transport, and application. Superhydrides, with their promise of dense hydrogen packing and superconductivity, could help tip the scales.
Still, there’s work to be done. Scaling up production, lowering synthesis pressures, and stabilizing these materials at ambient conditions remain key hurdles. But thanks to this new machine learning framework, those hurdles now seem surmountable rather than speculative.
This isn’t just a milestone for superhydrides—it’s a turning point for how we discover materials themselves.
A New Era for Material Discovery
In the end, this study does more than solve a single problem. It redefines how problems can be solved. By trusting algorithms trained on the laws of quantum mechanics and letting them explore chemical space beyond human reach, researchers have unlocked a new mode of scientific exploration.
The tools that once predicted weather or sorted cat photos are now predicting how matter reshapes itself under the weight of worlds.
We are witnessing the birth of a new kind of alchemy—one where silicon brains help human hands forge the materials of tomorrow. And in this collaboration between man and machine, the once-impossible dream of taming superhydrides may become our next great scientific reality.
Reference: Ryuhei Sato et al, Surface melting–driven hydrogen absorption for high-pressure polyhydride synthesis, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2413480122