All around us, carbon dioxide drifts invisibly in the air—a silent specter woven into the breath of engines, the chimneys of factories, and the atmosphere itself. Year by year, we pump more of it skyward, trapping heat, twisting the planet’s weather into unfamiliar shapes, and inching closer to climate calamity. For decades, scientists have searched desperately for ways to tame this relentless gas, to transform it from planetary poison into something useful, even precious.
At the heart of this quest lies a radical vision: what if we could capture carbon dioxide and turn it into fuel—a sustainable alchemy that not only locks away carbon but powers the engines of civilization?
A team of researchers affiliated with the Ulsan National Institute of Science and Technology (UNIST) in South Korea has just taken a thrilling step closer to that dream. In a breakthrough that could ripple through both industry and environmental policy, they have devised a novel way to convert CO₂ into methanol, one of the world’s most versatile chemicals and a promising fuel for the future.
The Unsung Hero: Methanol
Methanol is no stranger to the chemical world. It hides in plain sight, a ghost behind countless materials that shape our lives. From plastics to synthetic fabrics, solvents to pharmaceuticals, methanol is the quiet workhorse of modern industry. And beyond its industrial uses, it beckons as a liquid fuel and hydrogen carrier—easy to store, transport, and integrate into existing infrastructure. In the era of hydrogen economies and fuel cell vehicles, methanol’s star shines ever brighter.
But as alluring as methanol’s promise may be, producing it sustainably has remained a stubborn challenge. Traditionally, methanol is made from natural gas through processes that themselves emit carbon dioxide. Even emerging methods of converting captured CO₂ into methanol have stumbled over practical hurdles. They often yield messy mixtures full of unwanted byproducts like hydrogen and methane, demanding expensive purification. The dream of clean methanol production has remained just out of reach.
A Catalyst Like No Other
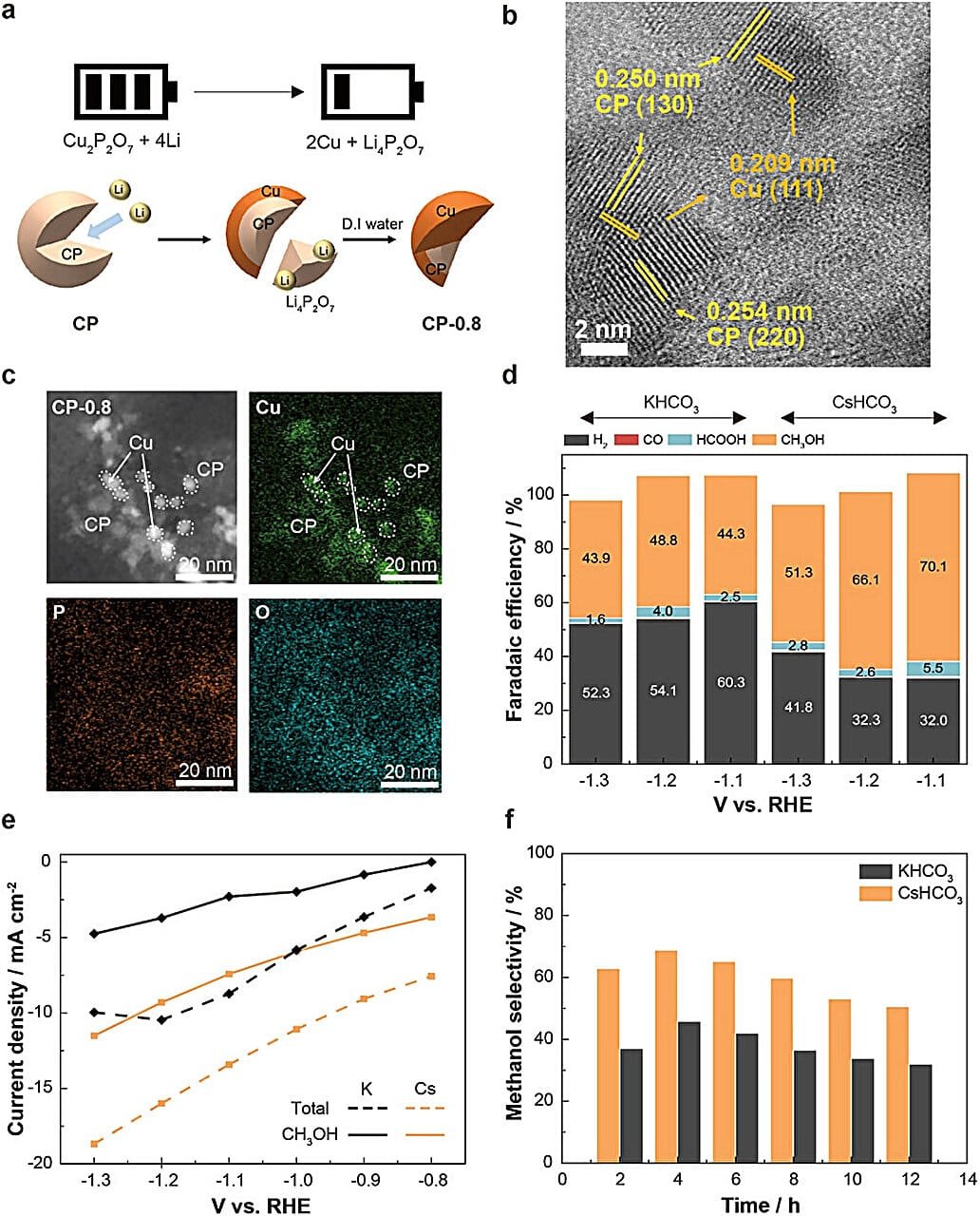
Led by Professor Jungki Ryu from UNIST’s School of Energy and Chemical Engineering, in collaboration with Professors Jongsoon Kim of Sungkyunkwan University and Aloysius Son of Yonsei University, the research team has cracked open a new possibility. In a paper published in Advanced Materials, they unveiled a catalyst that could change the entire game.
Their weapon of choice is copper—a humble, earth-abundant metal long studied for CO₂ reduction. Yet copper has always had a frustrating habit: it’s good at reducing CO₂ but too promiscuous, spawning side reactions that churn out other molecules instead of pure methanol. Most copper catalysts hover at a methanol selectivity of only 10% to 30%. Higher selectivity typically requires expensive precious metals like palladium or platinum, putting a sustainable price tag far out of reach.
The team’s breakthrough is astonishing: a copper-based catalyst achieving methanol selectivity as high as 70%. In the fiercely competitive world of catalytic chemistry, that number is seismic. It rivals—and in some cases surpasses—the performance of far costlier precious metal catalysts.
A Molecular Puzzle with Atomic Precision
So what’s the secret? The answer lies in architecture.
The scientists engineered a unique material where copper(I) pyrophosphate (Cu₂P₂O₇) nanoparticles are tightly fused with pure metallic copper. Picture a puzzle where each piece clicks perfectly into place. This intricate structure stifles the side reactions that usually plague copper catalysts, especially the unwelcome production of hydrogen. As a result, the reaction focuses its energy on forging methanol with striking efficiency.
Crafting this puzzle wasn’t just clever—it was innovative. The researchers took inspiration from an unexpected realm: lithium-ion batteries. In a typical battery, discharging causes metal ions to shuttle back and forth, changing the chemical structure of the electrodes. Harnessing this principle, the team passed an electric current through an electrode containing copper pyrophosphate. Part of it transformed into metallic copper while seamlessly entwining with the remaining pyrophosphate. The two materials merged into a composite that would have been nearly impossible to create by conventional chemical methods.
Equally impressive is how simple the final steps are. Once the catalyst forms, any leftover materials wash away in water—a gentle finishing touch that makes the manufacturing process surprisingly clean and scalable.
A New Road to Methanol
Yet the researchers’ discoveries did not stop with crafting a better catalyst. In the delicate dance of molecules on the catalyst’s surface, they observed a pathway few expected.
Traditionally, the conversion of CO₂ to methanol involves carbon monoxide (CO) as an intermediate. But in this new system, the researchers found that the catalyst first transforms CO₂ into formic acid (HCOOH), which then undergoes further reactions to yield methanol. This alternate route opens entirely new scientific frontiers. It suggests that methanol synthesis might be coaxed through multiple avenues, each with different energy requirements and efficiencies.
Understanding these mechanisms could spark a new wave of catalyst designs, each tailored to exploit the most efficient pathways and unlock higher yields of methanol from CO₂.
Bridging Lab Dreams to Industrial Reality
Professor Ryu emphasizes the real-world significance of this development. Methanol, he notes, is consumed worldwide in the millions of tons each year—a scale that makes any sustainable production pathway potentially transformative for both industry and climate change.
Their copper catalyst, made from inexpensive, abundant materials, offers a compelling alternative to rare and costly precious metals. Its high selectivity and current density push it closer to industrial deployment. Moreover, the clever use of battery-inspired techniques hints at manufacturing processes that could scale to meet global demand.
Professor Ryu is already looking ahead. He envisions expanding electrode areas and integrating entire systems capable of continuous operation. The dream is not merely scientific curiosity, but a commercially viable technology that could help rewrite humanity’s relationship with carbon emissions.
An Alchemy for the Climate Age
In the soft glow of laboratory lights, surrounded by whirring machines and precise instruments, scientists like Ryu and his team are performing a modern kind of alchemy. They are transmuting a greenhouse villain into a liquid ally—turning carbon dioxide, the gas haunting our future, into methanol, a fuel and feedstock brimming with possibility.
Their work radiates hope in a world often shadowed by climate despair. It suggests that the same ingenuity which propelled us into the industrial age might yet chart a sustainable path forward. With catalysts like these, the air itself could become a resource, not just a dumping ground for our waste.
The journey from lab bench to refinery floor will still require time, investment, and engineering finesse. Yet with each step forward, the vision sharpens: a future where we don’t merely fight carbon dioxide but reshape it into building blocks for a cleaner, more resilient civilization.
In this unfolding story of science and sustainability, the copper catalyst from UNIST stands as a symbol of possibility—a shining puzzle piece in humanity’s grand effort to harmonize progress with the fragile rhythms of our planet.
Reference: Hyunwoo Kim et al, Selective Electrosynthesis of Methanol from CO2 Over Cu/Cu2P2O7 Via the Formate Pathway, Advanced Materials (2025). DOI: 10.1002/adma.202501021