In a groundbreaking study that pushes the boundaries of material science, researchers from Kumamoto University and Nagoya University have unveiled a new class of metal-organic frameworks (MOFs) that may revolutionize applications in energy storage, molecular sensing, and spintronic devices. By introducing uniquely shaped molecules into the molecular framework, the team has created high-quality two-dimensional (2D) crystalline materials with rare electronic and magnetic properties—earning them a new classification altogether: 2.5-dimensional MOFs.
This innovative research, published in the Journal of the American Chemical Society, marks a significant step forward in a field that has long been hampered by challenges in crystal growth and the elusive link between structure and function.
Reimagining the Architecture of 2D Materials
Metal-organic frameworks are hybrid materials constructed from metal ions and organic molecules. Their porous, tunable structures have drawn interest for a wide range of uses—from gas storage and chemical sensing to catalysis and electronics. However, developing 2D conductive MOFs with precise, controllable properties has been notoriously difficult. Flat organic ligands tend to promote rapid stacking, leading to small, disordered crystals that are difficult to study in detail.
To break this bottleneck, Associate Professor Zhongyue Zhang of Kumamoto University, in collaboration with Professor Kunio Awaga’s team at Nagoya University, turned to a molecule long admired for its distinctive shape: triptycene. Unlike flat linkers, triptycene has a rigid, three-dimensional geometry resembling a paddlewheel or a propeller. This unique structure suppresses the stacking interactions that typically hinder the growth of high-quality 2D crystals.
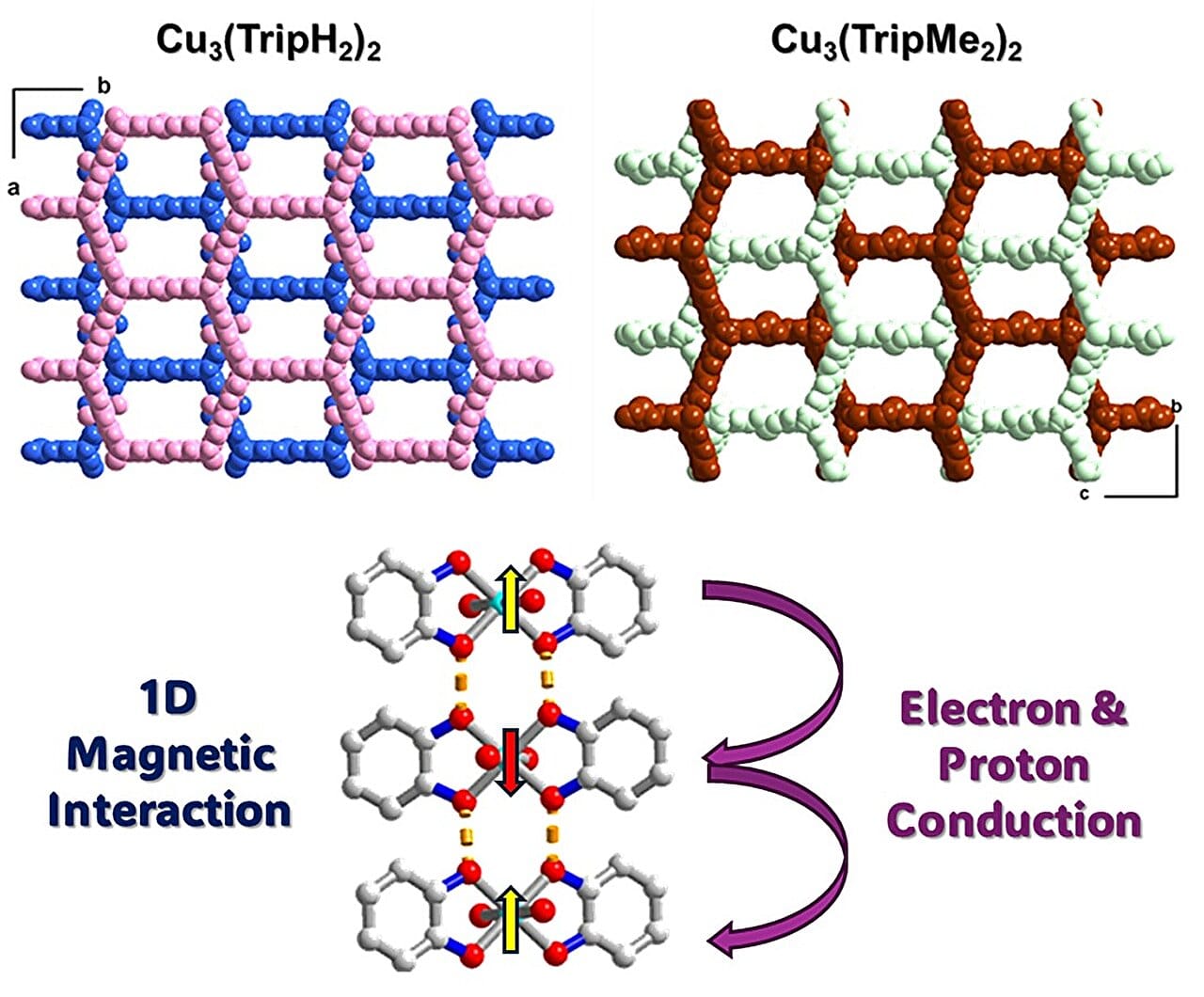
By slowing down the growth process using a sealed glass tube diffusion method—a stark departure from conventional solvothermal techniques—the team successfully synthesized two new MOFs: Cu₃(TripH₂)₂ and Cu₃(TripMe₂)₂. These crystals reached unprecedented sizes of over 0.3 mm, allowing for single-crystal X-ray diffraction and a rare opportunity to deeply analyze their internal structure and electronic behavior.
Cracking the Code of Conductivity and Magnetism
The success of these crystals did more than provide beautiful geometric structures—it opened a window into the microscopic workings of MOFs at a level of detail seldom achieved.
One of the most surprising discoveries came from the protonation state of the molecules. The research revealed that the catechol groups, which bond to the copper ions, remain fully protonated. This is a striking departure from most known MOFs, where these sites tend to lose protons. Not only was this unexpected, but it was also experimentally verified, offering the first direct evidence of how protonation can stabilize the structure through hydrogen bonding between the MOF layers.
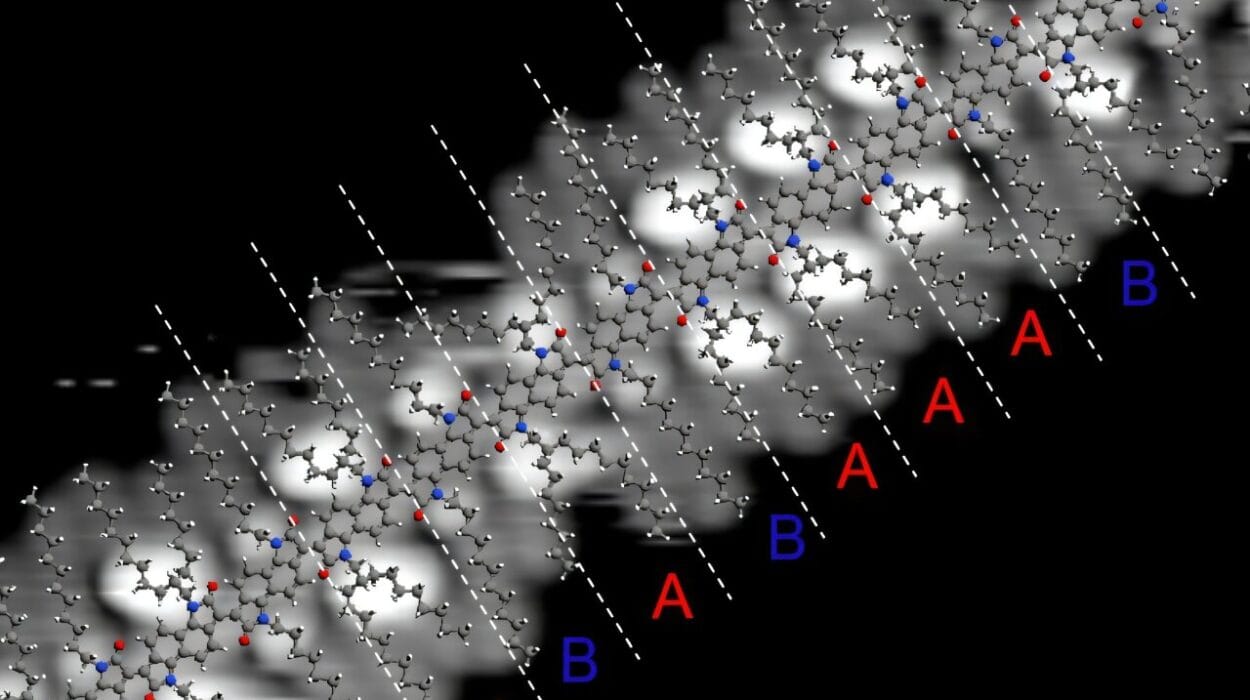
Even more intriguing were the directional properties of the crystals. Electron and proton conductivity measurements showed that charge moves much more efficiently vertically along the a-axis than in the horizontal layers. This is the opposite of what most 2D MOFs exhibit, where conductivity tends to flow within the plane. The findings suggest a cooperative hopping mechanism, where both electrons and protons travel between the branches of the triptycene structure like dancers moving in sync across a spiral staircase.
To understand the magnetic behavior of these materials, the team employed electron paramagnetic resonance (EPR) and magnetization measurements. These revealed one-dimensional antiferromagnetic coupling—a state where magnetic moments align in alternating directions—along the same vertical direction as the high conductivity. Such behavior stands in stark contrast to other 2D MOFs, where magnetic frustration often disrupts orderly spin alignments.
What’s especially remarkable is that these strong electronic and magnetic interactions occur without continuous bonding across layers. The layers are structurally separated, yet behave as if they are electronically connected. It’s a paradox that challenges our understanding of dimensionality in materials—and led the researchers to coin a new term: 2.5-D MOFs.
A New Paradigm for Functional Materials
The implications of this discovery are vast. For one, it demonstrates that a simple change in molecular geometry—from flat to 3D-rigid linkers—can profoundly alter the behavior of an entire class of materials. More than that, it provides a practical path forward in an area of research that has long been constrained by the inability to grow large, analyzable crystals.
“By enabling high-quality single crystals, we not only clarified fundamental structure–property relationships but also unlocked new potential for next-generation MOFs in real-world devices,” said Professor Zhongyue Zhang of Kumamoto University.
The findings open the door to multifunctional applications across several cutting-edge domains. In gas and molecular sensors, for instance, the directional proton conductivity could enable faster, more accurate detection mechanisms. In electrochemical energy storage, such as zinc-ion batteries, the ability to tune both electronic and proton transport could lead to higher-capacity, longer-lasting devices. And in spintronics—the emerging field that uses electron spin in addition to charge for data storage and logic—these materials could serve as the basis for low-dimensional magnetic systems with customizable behaviors.
Moreover, the discovery offers new tools for quantum information science. The intricate interplay of magnetic and conductive properties in a layered-yet-connected system could serve as a model for quantum entanglement, coherence, and other quantum phenomena.
Looking Ahead: The 2.5-D Frontier
The concept of “2.5-dimensionality” is more than just a catchy phrase. It reflects a profound shift in how scientists might design materials in the future—blending the accessibility and tunability of 2D materials with the emergent behaviors of more complex systems.
This new class of MOFs suggests that researchers no longer need to choose between order and functionality, or between scale and performance. By controlling molecular geometry at the atomic level, it is now possible to create crystals that grow slowly, stay stable, and still exhibit advanced properties that respond dynamically to electric and magnetic stimuli.
With further research, it may become possible to generalize this approach to other metal-organic frameworks using different metals and ligands, creating a library of 2.5-D materials tailored for specific functions—from wearable electronics to high-efficiency solar fuels to neuromorphic computing platforms.
As researchers continue to explore the boundaries between structure and performance, this work from Kumamoto and Nagoya stands as a testament to the power of creative molecular design, and how even the smallest structural tweaks can have earth-shaking implications.
In the end, this isn’t just about a new type of material—it’s about rethinking how we build the future from the molecular level up.
More information: Qi Chen et al, Triptycene-Based 2.5-Dimensional Metal–Organic Frameworks: Atomically Accurate Structures and Anisotropic Physical Properties from Hydrogen-Bonding Bridged Protonated Building Units, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c08703