Plastics that conduct electricity may sound like science fiction, but they’re very real—and they’re shaping the future of flexible electronics, wearable sensors, and even sustainable energy devices. These materials, known as conjugated polymers, are celebrated for being lightweight, inexpensive, and printable onto bendable surfaces. But now, a new study has revealed a hidden flaw that could be quietly undermining their performance at the molecular level.
In a paper recently published in Nature Communications, an international team of scientists has exposed critical weaknesses in the way these polymers are made—specifically, a widely used process called aldol condensation. Though this method is prized for being metal-free, eco-friendly, and scalable, the new research shows it may also be introducing structural defects that limit the material’s ability to conduct electricity or efficiently convert heat into energy.
Green Chemistry, Unexpected Consequences
The allure of aldol condensation lies in its simplicity and sustainability. It doesn’t rely on expensive or toxic metals, which makes it a darling of green chemistry. It’s flexible in terms of the molecular designs it can support and can be scaled up for commercial production. Yet, this “clean” chemistry may not be as flawless as previously thought.
“The aldol condensation process can create defects in the polymer sequences, like missteps in a molecular dance,” said Professor Giovanni Costantini, senior author of the study from the University of Birmingham. “These can disrupt the flow of electrons through the material, reducing efficiency and reliability in devices.”
In other words, the very method meant to simplify and green the production of electronic polymers might be introducing subtle imperfections that degrade the final product—imperfections that had flown under the radar until now.
Seeing the Unseen: Imaging Molecules One by One
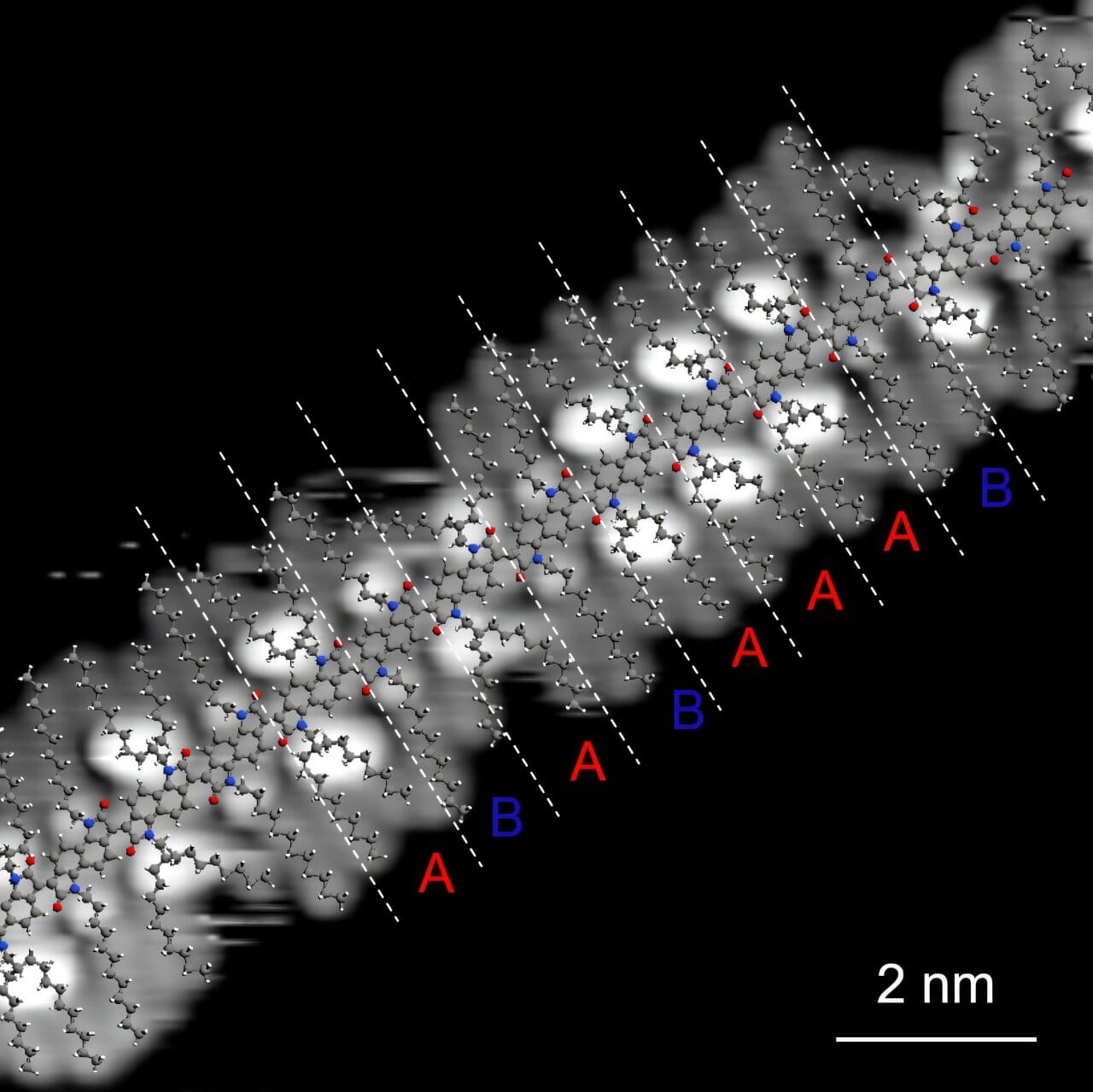
Detecting such elusive flaws is no easy task. Conventional techniques used to analyze polymer structures simply aren’t sensitive enough to pick up these misalignments. So the researchers turned to a pair of powerful technologies: scanning tunneling microscopy (STM) and electrospray deposition (ESD).
STM allows scientists to visualize individual molecules on a surface with near-atomic precision. When combined with ESD—a technique that gently deposits molecules onto a surface—it becomes possible to map out how each building block of a polymer chain is connected.
Using this high-resolution approach, the team examined four different polymers synthesized via aldol condensation. What they discovered was eye-opening.
The Two Faces of Polymer Defects
The study identified two primary types of defects within the polymer chains:
- Coupling Defects: Imagine a train where some cars are facing the wrong way or are joined at the wrong angle. These are coupling defects—errors in how the polymer units connect, resulting in kinks or breaks in the chain. In a conductive polymer, such disruptions can block or scatter the flow of electrons.
- Sequence Defects: These are more like putting two of the same puzzle pieces in a row when they’re meant to alternate. The overall structure might still resemble a proper chain, but the order of units is scrambled. This too can impact how electrons move through the material and how it interacts with light or heat.
These flaws may seem minor, but in the finely tuned world of molecular electronics, they can be the difference between a revolutionary material and a disappointing one.
Rethinking “Perfect” Plastic
What’s especially striking is that these defects aren’t rare—they’re baked into the material during synthesis. And they’ve likely gone unnoticed for years, simply because no one had looked this closely.
But the researchers didn’t stop at identifying the problem—they also sought solutions. By redesigning the chemical building blocks and purifying them more rigorously before polymerization, they were able to dramatically reduce the number of defects.
One strategy involved using aldol condensation only in the early stages to make small, well-defined molecules, which were then stitched together using a different, more precise method. This hybrid approach resulted in significantly cleaner, more consistent polymer chains.
“This is a major step forward in understanding how to make better-performing, more sustainable materials for electronics,” said Professor Costantini. “It shows that even green chemistry needs careful control to deliver the best results.”
The Stakes for Next-Generation Technology
Why does this matter? Because conjugated polymers aren’t just a lab curiosity—they’re already being used and tested in emerging technologies that promise to change how we interact with the world. Flexible solar panels, biosensors embedded in clothing, lightweight batteries, and energy-harvesting devices all rely on these kinds of materials.
If those polymers are full of microscopic defects, it could mean shorter device lifespans, lower efficiency, and inconsistent performance. In some cases, it might prevent promising innovations from ever reaching the market.
Moreover, the environmental advantages of aldol condensation—avoiding rare, costly, or toxic metals—depend on the resulting material being truly competitive. If it can’t match or exceed the performance of more traditional materials, its “green” appeal won’t be enough.
This new research offers a path forward: keep the benefits of green chemistry, but don’t overlook the microscopic imperfections it may introduce.
Zooming In to Advance the Future
The ability to see and understand these flaws at the molecular level represents a turning point in how scientists approach the design of next-generation electronics. It opens the door to smarter, more tailored materials—plastics that don’t just look like they work, but perform with precision, reliability, and sustainability.
This study also reminds us that progress often lies not in bold new inventions, but in looking more closely at what we already think we understand. Sometimes the smallest cracks in a material reveal the biggest opportunities for improvement.
As the electronics of the future become more flexible, more wearable, and more eco-friendly, research like this will be essential to ensuring they’re also robust, efficient, and built to last.
And as Professor Costantini puts it, “The beauty of science lies in its ability to uncover hidden truths. Even in something as seemingly mundane as a plastic film, there are secrets waiting to be found—and those secrets can change everything.”
More information: Xiaocui Wu et al, Revealing polymerisation defects and formation mechanisms in aldol condensation for conjugated polymers via high-resolution molecular imaging, Nature Communications (2025). DOI: 10.1038/s41467-025-62221-y